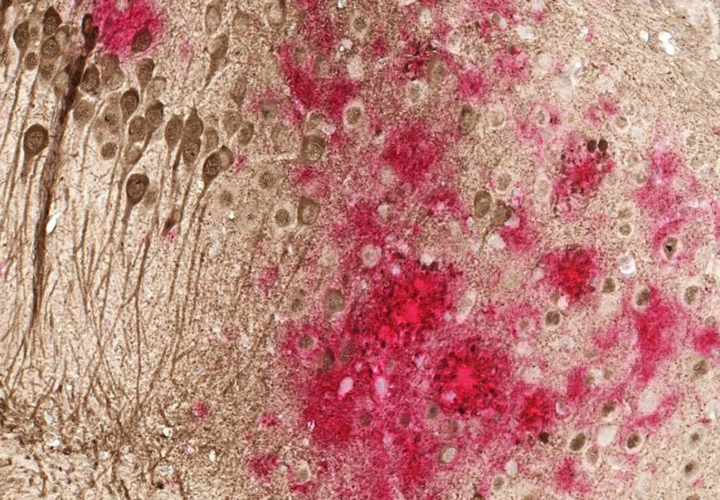
For the first time, researchers mapped the molecular structure of a beta-amyloid protein aggregate, or toxic proteins that accumulate in the brain and lead to Alzheimer's symptoms.
For the first time, researchers mapped the molecular structure of a beta-amyloid protein aggregate, or toxic proteins that accumulate in the brain and lead to Alzheimer’s symptoms.
Sometimes, beta-amyloid proteins turn into chains, or fibrils, causing cell death. The team of researchers from Binghamton University and the University of Colorado Denver used high-resolution solid-state nuclear magnetic resonance spectroscopy, or a device that allowed them to look at the chemical characteristics of these beta-amyloid fibrils.
The researchers discovered that the fibrils not only have large variations when it comes to the molecular structure of amyloid proteins in the brain, but also acted as “seeds” that generated additional fibrils, causing cell death and likely increasing Alzheimer’s risk.
The team is also investigating how differences between certain fibrils affect their “seeding abilities” and toxic impact on cells.
“This work describes a molecular structural model for a pathologically relevant beta-amyloid fibril variant,” said Wei Qiang, assistant professor of biophysical chemistry at Binghamton University. “We showed that this variant could lead to rapid seeding of new amyloid fibrils, which potentially contributes to the spreading and amplification of amyloid deposition in human brains.”
Earlier this week, researchers from Technische Universität München in Germany found that beta-amyloid prevents glutamine, a chemical that neurons use to send messages to each other, from moving past the gap between connected neurons. The scientists believe this process may also kickstart cellular dysfunction in Alzheimer’s.
While recent drug trials targeting beta-amyloid have failed, some researchers argue that pharma needs to test these drugs on participants before Alzheimer’s symptoms appear. This way, researchers or doctors could stop the disease in its tracks before people experience memory loss.
In this case, researchers think that mapping the structure of amyloid fibrils could provide them with a better understanding of how these proteins cause Alzheimer’s and with more detailed treatment options.
“Approximately 10 percent of Alzheimer’s cases result from familial mutations,” said Qiang. “The other 90 percent of cases are caused by misfolded wild-type amyloid proteins. We need to understand the molecular basis of the disease pathology. In doing so, we might one day create drugs that prevent the degenerative effects of the disease.”
Access the full study here.



Sounds like something an 80 year old healthy person could do.r