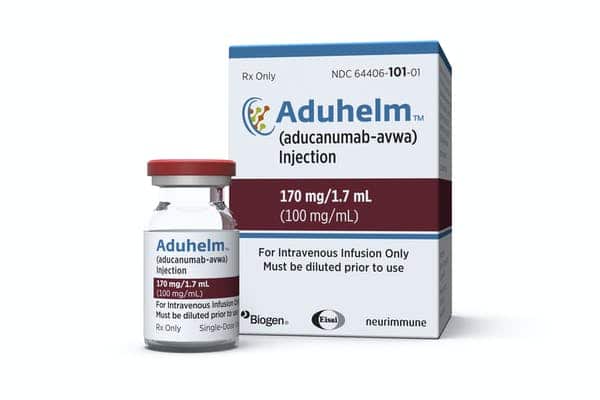
UPDATE: 3 March 2024, 8:22 P.M. ET. In February 2024, Biogen took Aduhelm off the market, citing financial concerns. Although the drug did receive accelerated, conditional FDA approval for the treatment of early Alzheimer’s disease in 2021, it is no longer available to new patients. The company announced it would sunset trials in May 2024 and cease supplying the drug to current patients in November 2024.
Will Medicare cover Alzheimer’s drugs like Aduhelm? That decision-making process is underway.
This week, the U.S. government embarked on the formal review process to determine whether Medicare will establish a national coverage policy for monoclonal antibodies (MABs) targeting amyloid for the treatment of Alzheimer’s.
This analysis, known as a National Coverage Determination (NCD), includes Biogen’s controversial Alzheimer’s drug, Aduhelm, which is on the market now. But it will also apparently apply to drug candidates still pending FDA approval. If Medicare denies coverage, this could force drugmakers currently developing anti-amyloid drugs similar to Aduhelm to approach pricing entirely differently.
MABs are laboratory-made proteins that mimic the immune system’s defensive abilities. The approach of targeting of beta-amyloid plaques to fight Alzheimer’s — which stems from a theory about the disease’s pathology, known as the amyloid hypothesis — is the leading approach in drug development, though it is not completely proven. Aduhelm’s approval, however, gave this approach a big boost by the FDA, and since, the agency has expedited the review process for two other MABs, giving “breakthrough status” to Eli Lilly & Co.’s donanemab and Biogen and Eisai’s lecanemab.
The Centers for Medicare & Medicaid Services (CMS) announced Monday that it is weighing whether the program will cover MABs, and that as part of the NCD, a 30-day public comment period is now in swing. CMS plans to host two public listening sessions in July to provide an opportunity for public input. They will issue their proposed decision within six months, followed by a final decision within nine months.
“NCDs are program instructions developed by CMS to describe the nationwide conditions for Medicare coverage for a specific item or service,” the CMS press release explains. “This NCD analysis will be applicable to national coverage considerations for aducanumab, which was recently approved by the Food and Drug Administration (FDA), as well as any future monoclonal antibodies that target amyloid for the treatment of Alzheimer’s disease.”
According to the AARP, Medicare currently supports some 44 million beneficiaries — about 15 percent of the U.S. population (one in 10 of those beneficiaries relies solely on Medicare for their healthcare coverage). As the U.S. general population ages, the total number enrolled is expected to rise to 79 million by 2030.
“Alzheimer’s is a devastating illness that has touched the lives of millions of American families and as CMS opens our National Coverage Determination analysis, we invite interested stakeholders to participate,” CMS Administrator Chiquita Brooks-LaSure said in the statement. “We want to consider Medicare coverage of new treatments very carefully in light of the evidence available. That’s why our process will include opportunities to hear from many stakeholders, including patient advocacy groups, medical experts, states, issuers, industry professionals, and family members and caregivers of those living with this disease.”
For now, coverage determinations for Aduhelm (aducanumab) are being made at the local level by Medicare administrative contractors across the country. In terms of a national coverage policy, CMS stated its analysis will determine whether the drug meets Medicare’s requirements that items or services be “reasonable and necessary for the diagnosis or treatment of illness or injury.”
Want to learn more about clinical trials
for Alzheimer’s and dementia?
Check out the Lilly Trial Guide.
At least for Aduhelm (aducanumab), this may be a tough hurdle, as its efficacy data is notoriously murky, and the FDA has required Biogen to continue phase 4 drug trials to further establish this efficacy. Those may be ongoing for years.
The first drug of its kind to be FDA-approved, Aduhelm’s estimated cost has been criticized widely for being sky-high, and is even the subject of calls for investigation by members of Congress.
According to Biogen, the estimated cost is based upon an infusion that patients will receive once every four weeks, with dosage determined by a person’s weight. If the drug recipient weighs what Biogen pegged as average adult weight, 163 pounds, each infusion will cost an average of $4,312. For 12 infusions per year, that makes the cost some $56,000 annually.
Experts say affording the drug could be especially complicated for people without access to quality healthcare and to the very large population of people with Alzheimer’s on Medicare. Further, it will be a burden on the Medicare system at large.
According to the AARP, Medicare currently supports some 44 million beneficiaries—some 15 percent of the U.S. population. One in 10 beneficiaries relies solely on Medicare for health care coverage. As the U.S. general population ages, the total number enrolled is expected to rise to 79 million by 2030.
“I think the hard thing is that people have longed for a drug that would treat or cure Alzheimer’s and have a lot of hope that this might be the drug,” Tricia Neuman, senior vice president of the Henry J. Kaiser Family Foundation and executive director of its program on Medicare policy, told Being Patient, “but this could be a high price to pay for a drug that may or may not have much of an effect.”