The FDA will soon make its approval decision on Biogen drug aducanumab, an Alzheimer's treatment with high potential in reducing clinical decline.
In yet another encouraging step toward the development of what could be the first drug approved for Alzheimer’s disease, the Federal Drug Administration today said it would decide whether to approve Biogen’s aducanumab drug no later than March 7, 2021.
If approved, aducanumab would become the first drug proven to slow the cognitive decline associated with Alzheimer’s disease. In announcing its decision, the FDA said that it would grant priority review to the Biogen application and added that it would try to decide on the application before the March target date.
“We believe that aducanumab marks the beginning of a new era of potential treatments for Alzheimer’s disease that will inspire even more discovery and innovation,” Michel Vounatsos, chief executive of Biogen, said in a news release.
Eisai Co., which is co-developing aducanumab, also welcomed the FDA’s action.
“Reducing clinical decline and maintaining the ability to live an independent life for as long as possible are things that people living with Alzheimer’s disease and their families value in a potential treatment,” Haruo Naito, Eisai chief executive officer, said in the news release.
The path to today’s FDA decision has been quite twisted. Initially introduced in early stage trials in 2016, aducanumab entered its Phase 3 trials with high hopes for success. But in March 2019, Biogen and Eisai stunned the Alzheimer’s community when they abruptly halted the end of the drug’s development after aducanumab failed a “futility analysis.”
The companies then reversed themselves last October, saying that late-arriving data had actually shown improvements for people living with mild cognitive impairment or very early Alzheimer’s. And in December, at a packed conference in San Diego, Biogen said that patients who received aducanumab experienced significant slowing of decline on measures of cognition and functions such as memory, orientation and language.
Biogen and Eisai officially sought FDA review of aducanumab last month, submitting an application of more than 2.5 million pages.
The next step in the FDA process is expected to be the appointment of an advisory committee, a group of outside experts who would review the aducanumab application. No date has been set for the formation of the committee.
Biogen’s stock rose on today’s action by the FDA, although many analysts are still skeptical. “The bottom line is, the FDA standard of approval is substantial evidence of efficacy and the cumulative data for aducanumab falls really far of this standard,” Baird analyst Brian Skorney told Fierce Biotech.
But Brian Yee, an analyst with Jefferies, was more optimistic, saying that the FDA’s decision to do a priority review suggests that the agency might be “comfortable with the totality of the data, recognizes high unmet need and really wants to get an Alzheimer’s drug approved.”
The Alzheimer’s Association welcomed the new development. “This is an important next step in the process, and the Alzheimer’s Association looks forward to the results of the FDA’s review,” said Maria C. Carrillo, the Association’s chief science officer. “If this drug is approved, our primary effort will be to ensure access to the drug for everyone that could benefit from it.”
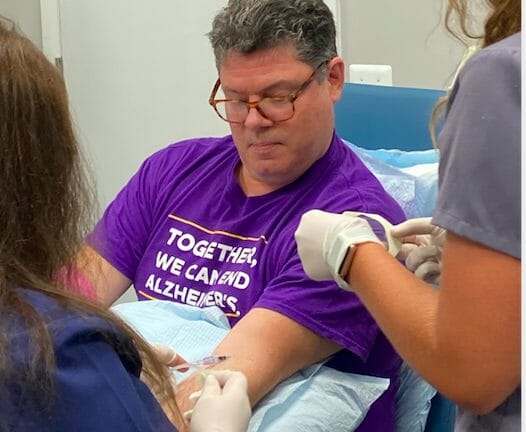
Phil, who is living with early-onset Alzheimer’s, is a participant in the aducanumab trial.


I would live to get my husband, age 65, recently diagnosed. Mild cognitive decline but I see progressively worsening every week. How can we get approval for this drug?? In a hurry.