“We are looking for older volunteers with MCI or mild dementia due to AD or Lewy body dementia, and healthy older individuals, who wish to partner with us to advance the field. Current treatments for AD are clearly inadequate.” -Dr. R. Scott Turner, Georgetown’s Memory Disorders Program Director
A cancer drug, nilotinib, is the first oral treatment to be found to lower amyloid burden in the brain for people living with Alzheimer’s disease.
Now, a Georgetown University Medical Center clinical trial has made new progress in testing it as a possible treatment for Alzheimer’s.
Nilotinib, brand name Tasigna®, is an FDA-approved treatment for chronic myeloid leukemia. Originally, researchers at Georgetown had decided to experiment with the treatment because they knew it triggered the “garbage disposal” machinery inside neurons — a process known as autophagy — to clear out Tau, beta-amyloid and other toxic proteins — hallmarks of Alzheimer’s and other neurodegenerative diseases.
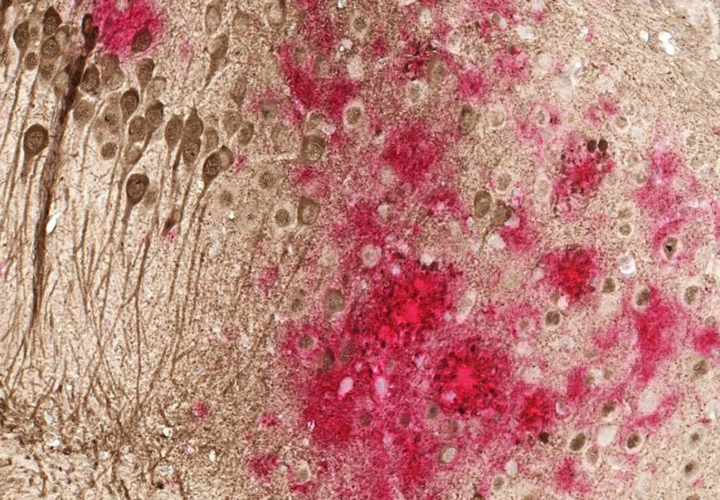
Indeed, it appears to be able to penetrate the blood-brain barrier and aid in the clearance of beta-amyloid plaques and Tau tangles in neurons in the brain. Using brain imaging, the researchers determined that the amyloid burden in those who took the drug was lower than those in the placebo group. But the most recent trial had a very specific objective: to determine whether it was safe and well-tolerated for its target audience.
According to Dr. Charbel Moussa, director of the Georgetown Translational Neurotherapeutics Program and a senior author on the study, these Phase 2 studies met their primary objectives, finding that nilotinib is safe and tolerated by people living with Alzheimer’s disease.
While this is the case, adverse events — particularly mood swings, agitation and irritation — were noted at certain higher dosages. One known possible side effect of nilotinib at higher dosages is irregular heart rhythms, so people with abnormal cardiac rhythms have been excluded from trials, and no cardiac effects have been observed any trial participants.
The drug has also shown promise, in past studies at Georgetown, for people living with Parkinson’s and Lewy Body dementia.
“The data across the PD, AD and LBD studies are consistent, and demonstrate that nilotinib enters the brain and it is a potential disease-modifying drug that significantly increases the level of brain dopamine and reduces neurotoxic protein levels, including amyloid plaques and toxic tau (tangles),” Moussa told Being Patient.
About the Nilotinib Trial
After careful screening, 37 people with mild dementia due to Alzheimer’s were randomized to either the placebo or nilotinib groups for the 12-month study. Some received a 150 mg daily dose of nilotinib for 26 weeks, followed by another 26 weeks of a 300 mg daily dose. Others received placebos, not disclosed to participants nor researchers.
The results of the small, phase II, randomized, double blinded, placebo-controlled study were published online today in Annals of Neurology.
This trial follows nearly six years of past research:
According to Alzforum, in November 2014, researchers at Georgetown began exploring the effects of nilotinib on people living with cognitive impairment due to Parkinson’s or Lewy Body dementia. They saw it was safe, tolerated, and that it effectively crossed into the brain, raising dopamine levels and improving motor and non-motor symptoms. A phase 2 trial, which introduced a placebo, began in 2017. Phase 2a investigated safety, with a secondary look at motor and cognitive symptoms, wrapping up in September 2019.
It wasn’t clear from the 2017-2019 Phase 2a study whether the drug improved motor or cognitive function over the placebo. The latest study sets a new course.
What’s Next for Nilotinib and Alzheimer’s?
“The goal of the study is to determine drug effectiveness, now that safety is proven,” Dr. R. Scott Turner, lead study investigator and director of Georgetown’s Memory Disorders Program told Being Patient.
When it comes to the timeframe for next steps in an Alzheimer’s nilotinib trial, the team said that a Phase 3 trial that will be larger, placebo-controlled, double-blind study in early Alzheimer’s, currently planned to commence in the next one to two years.
As with any clinical trial, there are a number of pieces to put in place: The team will review trial plans with the FDA; secure donations of the nilotinib and a matching placebo from the drug manufacturer; and seek funding from the NIH and other foundations to lead that nationwide, multi-center study.
Meanwhile, a nilotinib trial focused on participants living with Lewy Body is underway through August 2023.
For those interested in and eligible to participant in a study, a list of ongoing research studies at Georgetown, with contact information, is available at memory.georgetown.edu.
“We are looking for older volunteers with MCI or mild dementia due to AD or Lewy body dementia, and healthy older individuals, who wish to partner with us to advance the field,” Turner said. “Current treatments for AD are clearly inadequate.”


Will you open up the trial study for Nilotinib for LBD to locations across the United States?
Hello Connie, BeingPatient.com does not provide trial studies. We are a news media service. ~ Thank you.