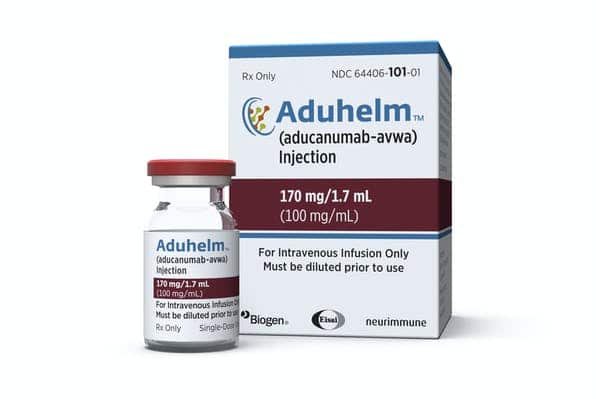
The FDA issued an update to Aduhelm’s label, now requiring a total of four MRIs during the first year of treatment to aid in the earlier detection and management of amyloid-related imaging abnormalities.
UPDATE: 3 March 2024, 8:30 P.M. ET. In February 2024, Biogen took Aduhelm off the market, citing financial concerns. Although the drug did receive accelerated, conditional FDA approval for the treatment of early Alzheimer’s disease in 2021, it is no longer available to new patients. The company announced it would sunset trials in May 2024 and cease supplying the drug to current patients in November 2024.
Biogen announced that the Food and Drug Administration (FDA) has updated the label for its anti-amyloid drug Aduhelm. These changes will add an additional two MRI scans within the first year of treatment. In total, four magnetic resonance imaging (MRI) scans will be advised.
The cost of an MRI scan typically falls somewhere between $375 to $2,850 in the United States. The national average was upwards of $2,000 as of 2014. These costs would be covered by Medicare for people participating in Aduhelm clinical trials. For everyone else, this adds to already hefty out-of-pocket costs. MRIs are only available at hospitals or radiology centers, and each one requires a referral from a doctor.
In its news release, Biogen wrote that the “label changes aim to further help doctors in identifying and managing patients, and aid in the earlier detection and management of amyloid-related imaging abnormalities (ARIA).”
What is ARIA?
ARIA is one of the potential side effects of Aduhelm which causes brain swelling or bleeding — but in many cases — is asymptomatic.
As regulators in Europe and Japan declined to approve the drug — in part due to the risk of ARIA — the Centers for Medicare & Medicaid Services also limited insurance coverage to patients in Aduhelm clinical trials. This comes on the heels of downsizing at Biogen as the company significantly reduces its production capacity of Aduhelm.
Biogen was also criticized for not reporting on clinical endpoints related to ARIA-associated changes in brain volume, in their submission to the FDA.
What is the risk of ARIA for Aduhelm patients?
According to data from Biogen’s Phase 3 trial, about one in three people taking the highest dose of Aduhelm developed ARIA. Only about three in a thousand people experienced severe side effects, which led to hospitalization and ongoing cognitive impacts. While experiencing symptomatic ARIA is rare, the symptoms usually include headache, confusion, dizziness, vision changes, and nausea.
The greatest risk factors for developing ARIA are past micro-hemorrhaging in the brain or carrying the ApoE4 gene. In addition, this side effect was more likely to occur in individuals taking higher doses of Aduhelm.
Since Aduhelm’s initial trials were not diverse, it is unclear whether the rate of ARIA would be higher in a more representative population.
Adam Brickman, a professor at Columbia University was worried by this data. “It’s hard to put a positive spin on the neuroimaging abnormalities,” he wrote on AlzForum. “We simply do not know the long-term consequences.”
Weighing the risks and benefits of Aduhelm for Alzheimer’s
Since the efficacy of Aduhelm remains controversial and the long-term consequences of ARIA are unknown, it is hard to weigh the risks and benefits. Lon Schneider, a professor at the University of Southern California, noted on Alzforum that even with the best surveillance practices, brain swelling can still be missed.
This is reported to have contributed to the death of a 76-year old woman during the clinical trial. However, according to the company, adding two additional MRI scans could help improve the surveillance of patients on the drug, allowing clinicians to spot and manage ARIA, even earlier.
“We believe the labeling updates further clarify how to appropriately manage patients treated with Aduhelm, with the goal of maximizing potential clinical benefits and mitigating adverse event risks,” said Maha Radhakrishnan, chief medical officer at Biogen.
The upcoming Phase 4 confirmatory trial will provide more data, allowing clinicians to better weigh the risk of ARIA against potential benefits of Alzheimer’s.