What if you could get an Alzheimer’s vaccine like you do for measles or chickenpox? Scientists at University of Texas Southwestern Medical Center are hoping that day isn’t too far off based on the results of a study using mice.
Researchers claimed to have found a vaccine that stopped the accumulation of the beta-amyloid and tau proteins in the brain, two of the biomarkers of Alzheimer’s and the two most popular targets for Alzheimer’s drugs.
If the onset of the disease could be delayed by even five years, that would be enormous for the patients and their families. The number of dementia cases could drop by half.
They tested the vaccine, delivered via a shot through the skin, on mice who have been genetically engineered to produce the plaques and tangles associated with Alzheimer’s. The shot is made up of DNA from Alzheimer’s proteins that can “teach” the body how to eliminate the proteins in the brain. The injection is called Aβ42, and it makes a three-molecule chain of the protein. The body then produces an antibody, which was shown to stop the build-up of both amyloid and tau.
“This study is the culmination of a decade of research that has repeatedly demonstrated that this vaccine can effectively and safely target in animal models what we think may cause Alzheimer’s disease,” said Dr. Roger Rosenberg, founding Director of the Alzheimer’s Disease Center at UT Southwestern. “I believe we’re getting close to testing this therapy in people.”
In the mouse study, the vaccine lowered beta-amyloid by 40 percent and tau by 50 percent.
This isn’t the first time anti-amyloid drugs have been tested. But in the past, they caused swelling of the brain. This vaccine triggers an immune response that doesn’t result in swelling.
If amyloid and tau are the cause of Alzheimer’s, this could be a potential therapy that could slash the number of people with the disease.
“If the onset of the disease could be delayed by even five years, that would be enormous for the patients and their families,” said Dr. Doris Lambracht-Washington, the study’s senior author. “The number of dementia cases could drop by half.”
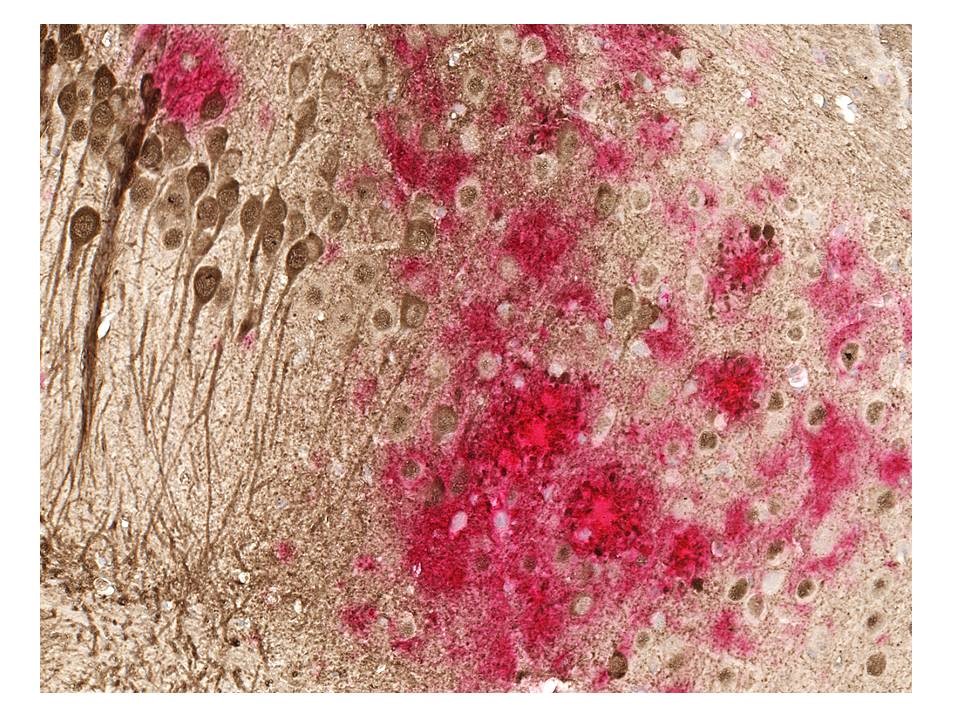
But some scientists don’t believe that beta-amyloid and tau are in fact the cause of Alzheimer’s. Some people have the proteins in their brain, but never get Alzheimer’s. Other scientists point to inflammation as the trigger for the disease.
“We know that inflammation has different phases–early on it can be protective against a threat by actively degrading it, but if the threat is not removed, then persistent inflammation actually causes cell death,” Professor Robert Richards, from the University of Adelaide’s School of Biological Sciences, told Being Patient.
However, if beta-amyloid and tau are behind Alzheimer’s, another hurdle would be to get this vaccine to people who are at risk before the disease starts to deteriorate the brain. Currently only a PET scan or a cerebrospinal fluid tap can determine whether Alzheimer’s is at play in the brain, though scientists are working on early diagnostic tests like an MRI, eye scan and even a neck scan.
“The vaccine would be offered to those over 60 years who show evidence of mild cognitive impairment or early Alzheimer’s disease based on a neurological examination, neuropsychological tests, clinical laboratory comprehensive metabolic profile tests, and abnormal levels of [beta-amyloid and tau] in the cerebrospinal fluid and increased accumulation of [beta-amyloid] and tau in a brain PET scan,” said Rosenberg. The patient would need to be cleared of other issues that might be causing memory loss, he added.
But scientists have high hopes for this vaccine. The mice whose plaques were cleared are currently undergoing cognitive tests to determine if reducing the beta-amyloid and tau also improved their cognition, said Rosenberg.
“It is expected that DNA Aβ42 trimer immunotherapy in a clinical trial will reduce both plaques and tangles in patients with [Alzheimer’s disease],” wrote the study authors.




My grandfather and father had alzaimers they are gone. I had a MRI done. They informed me that my brain is smaller then normal for my age. I forget alot. I wish i could try this vaccine. I am only 36 of age.
My children ages 37 and 39 both have Alzheimer’s. They inherited the PSEN1 gene from their father who also had Alzheimer’s and passed the day after his 43rd birthday. So far 6 of their family members have passed from the ages of 37 to 45from Alzheimer’s.
Do you know if there is genetic mutation in your family?
There is, in my family. How soon can humans be used for the clinical trials in the US?
I WOULD LIKE TO RECEIVE THIS VACCINE OR PARTICIPATE IN THE TESTING. Is there any information in that direction?
Hello
I would love for my sister to receive this treatment as she had been a caregiver for her
husband who has Parkinson’s while no one was aware that she had Alzheimer’s. Life isn’t fair, but I would love for her to have a chance to enjoy her grandchildren, who are 4 years old and love their memaw ever so much.
Thank you for considering her to the vaccine or a chance to participate in any clinical trial.
02/01/20. I would very like to be considered to participate in receiving this Vaccine, along with other tests.
I am male, 68 years of age and was diagnosed with very early onslaught Alzheimer’s at the age of 64.
I had the SPEC scan as my diagnoses along with other test.
Thank you for all the above information, I found it very informative, and wish you every success in future trials. Regards, Graham Harrison.
I am 81 years old and have the APOE4 gene, inherited from my mother, who lived the last 13 years of her life in an Alzheimers care facility. (She showed signs of the disease at around 75 years). Although I notice some memory loss in myself, especially word loss, my friends and children do not.
I would like to be in a trial, but I do not live close to a metropolitan area where I can easily drive to appointments. How can I sign up for a trial with the least amount of travel?
Thank you,
Teresa
My friend am 75 have just started renovare 500—google it—i have been on it only 3 weeks and effects are meant to take 2 to 6 months so its early days—-it costs 56.95 for a month in the uk—i think about 75 us dollars—-good luck
Patrick, Consult Your Physician – Being Patient does not give medical advice, nor is any information on the site intended to be prescriptive medical advice. If you have any questions about your health or the health of your loved one, please consult your physician for any and all medical-related questions. ~Thank you.