As of February 2024, here are the blood tests for Alzheimer's disease currently available to U.S. patients, how much they cost, how accurate they are.
Alzheimer’s blood tests: a new frontier in neurodegenerative diagnostics, getting the world a big step closer to earlier, more accessible Alzheimer’s diagnosis. But it’s a very new field, and while there are a number of blood tests already on the market, here are some things to remember: Alzheimer’s blood tests can’t give you a definitive Alzheimer’s diagnosis. Getting one of these blood tests could affect insurance coverage. So far, the tests aren’t standardized — and results will be hard to understand. And last but not least: If you do get a positive result, there’s no clear plan for what you should do next.
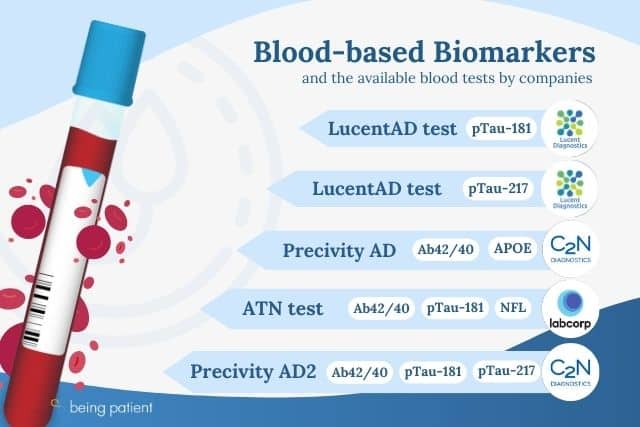
As of April 2024, there are at least eight different Alzheimer’s blood biomarker tests on the market, by four different companies. Some of these tests measure one specific biomarker while other tests incorporate multiple biomarkers at the same time. If you’re still curious about what’s out there, what exactly they test for, how the process works for patients and how much they cost, we rounded up all the answers for you.

C2N Diagnostics blood tests
C2N offers tests that are ordered by doctors to screen patients who are 55 years or older experiencing some symptoms of memory loss or cognitive decline. The company’s website states that they are not intended to be used in younger patients or for testing people who do not have any symptoms of the disease. The tests are available across 49 states, the District of Columbia and Puerto Rico. The company is working toward obtaining certification to permit providing the test in New York.
In contrast to many other competitors, C2N has published several research studies checking the validity of their blood tests and are currently in use across several clinical trials to screen participants and also track disease progress over time.
Joni Henderson, senior director of corporate communications for the company, told Being Patient that their tests are used alongside other information including “ neurological testing, medical and family history, clinical examination, and other evaluations” to help diagnose or rule out Alzheimer’s.
PrecivityAD™ test
Precivity AD is one of the first commercially available blood tests designed to measure the ratio of two forms of beta-amyloid proteins called Aβ42/40 in the blood as well as determine which form of the APOE gene you carry. Low levels of Aβ42/40 are associated with a higher risk of Alzheimer’s. It has to be ordered by a doctor, and is intended to be used to test people who are experiencing problems with their memory or thinking.
The APOE4 gene increases the risk of developing Alzheimer’s, though not for American Indians. A less common form called APOE2 may provide protection against developing Alzheimer’s. As early as last year, this blood test was used in large clinical trials to help screen for eligible participants. Another study conducted in collaboration with C2N Diagnostics found that Aβ42/40 performed equally well as a diagnostic in white people as well as in Black populations.
PrecivityAD2™ test by C2N Diagnostics
The AD2 test is a newer test from the company, released at the start of 2023, designed for patients aged 55 and older with signs or symptoms of mild cognitive impairment or dementia, who are undergoing evaluation for Alzheimer’s disease or other causes of cognitive decline.
It measures the Aβ42/40 ratio as well as the ratio of two other proteins which provide clues about tau levels in the brain — pTau-217 and npTau-217. A higher ratio of pTau-217/npTau-217 indicates a greater buildup of tau tangles in the brain. Then these two ratios are plugged into an algorithm that can tell doctors whether you are at risk of developing Alzheimer’s.
How do the tests work?
A neurologist will set up a lab appointment. The test will be sent to the laboratory where blood is drawn. After the blood draw, the sample is sent off to Precivity for processing.
How fast are results available?
The results are available within ten business days after blood is drawn.
How much do they cost?
The Precivity AD™ test costs $1,250, and AD2™ costs $1,450. They are not currently covered by insurance, but the company makes financial assistance available.
How accurate is are C2N’s Precivity tests?
The Precivity AD™ test correctly detects beta-amyloid plaques 92 percent of the time. It returns a false positive 23 percent of the time. That means you have a nearly one-in-four chance of being told you have Alzheimer’s pathology in your brain, when in fact, you don’t.
Precivity AD2™ test correctly detects beta-amyloid plaques 88 percent of the time, and it returns a false positive 11 percent of the time.

Quanterix and LucentAD
Quanterix publicly launched its first Alzheimer’s blood test at the end of July 2022, and since then has spun out a subdivision called Lucent Diagnostics to administer and process these tests. In October 2023, they launched another test which company CEO Masoud Toloue told Being Patient is more accurate. The company’s blood tests have also been used in at least 10 different clinical trials and studies.
LucentAD p-tau181 test
The company’s first blood test measures the levels of a protein called p-tau181. Higher levels of p-tau181 in the blood are linked to beta-amyloid pathology in the brain. A 2022 study found that pTau-181 may be inaccurate as a diagnostic biomarker for Black people.
LucentAD p-tau217 test
This test measures the levels of pTau-217 in the blood. Higher levels indicate a greater likelihood of beta-amyloid plaques in the brain. According to Toloue, it follows the stringent guidelines laid out in the draft of new diagnostic guidelines for Alzheimer’s disease.
“The test is capable of providing results to both rule out individuals with low risk of amyloid pathology and rule in individuals with high risk of amyloid pathology,” he said.
If someone’s pTau-217 measurement is below a cut off, it indicates a low likelihood of beta-amyloid plaques in the brain. If it is in an intermediate range, then it means that the person taking the test may have beta-amyloid pathology, and may require further testing or monitoring. If it is above this cutoff, it indicates an even higher risk of the presence of beta-amyloid pathology and along with other neurological or imaging tests, may be used to diagnose Alzheimer’s.
How does the test work?
A neurologist will set up a lab appointment. The test will be sent to the laboratory where blood is drawn. After the blood draw, the sample is sent off to Lucent AD for processing.
How fast are results available?
The results are available within ten business days after blood is drawn
How much does the test cost?
The test currently costs $300 and it is not covered by Medicare or other insurers at this time.
How accurate is the test?
The pTau-181 test correctly detects beta-amyloid plaques 90 percent of the time with a 44 percent rate of false positives. That means more than two out of five patients who take the test could have their ability to get insurance coverage could be compromised — even if they don’t actually have these Alzheimer’s biomarkers at all. The pTau-217 test detects beta-amyloid plaques 90 percent of the time and returns a false positive two percent of the time. Toloue mentioned that they are focusing on the newer pTau-217 test which is more accurate.
Labcorp’s Blood Tests
Labcorp has released two blood tests that measure biomarkers of Alzheimer’s pathology.
Labcorp’s ATN test
In October 2023, Labcorp launched the ATN blood test, which measures the levels of Aβ42/40, pTau-181, and a protein that indicates brain inflammation called NfL. The test is available for physicians to use in conjunction with a neurological exam, medical history, and other cognitive tests when screening for Alzheimer’s disease.
How does the test work?
A neurologist will set up a lab appointment at a Labcorp facility where the blood can be drawn and processed.
How fast are results available?
The results are available within ten business days after blood is drawn
How much does the test cost?
A spokesperson for LabCorp said that the testing may be covered in part by many insurance providers. It is currently not covered by Medicare or other insurers and costs $626 out-of-pocket.
How accurate is the test?
Labcorp said that the Aβ42/40 measurement accurately detects beta-amyloid in the brain 96 percent of the time and returns a false positive 13 percent of the time.
Labcorp’s pTau-217 test
In March 2024, Labcorp launched a blood test that measures the levels of pTau-217. The test was validated on a cohort of 200 patient samples but the results of this study were not published. When asked if there were any peer-reviewed studies comparing this test to other tools for detecting Alzheimer’s disease, a spokesperson provided citations to published research of other company’s blood tests.
How does the test work?
A neurologist will set up a lab appointment at a Labcorp facility where the blood can be drawn and processed.
How fast are results available?
The results are available within five to seven business days after blood is drawn
How much does the test cost?
The test costs $227 out-of-pocket. Labcorp told Being Patient that they are in discussions with health insurers about covering the test.
How accurate is the test?
The test will detect people with amyloid brain pathology correctly 95 percent of the time and will return a false positive 16 percent of the time.

Quest-AD Detect by Quest Diagnostics
The Quest-AD Test was the only test available for purchase online directly by patients and offered to anyone over the age of 18 with risk factors of Alzheimer’s — which includes a large chunk of the population — whether or not they have any symptoms. But as of March 12, 2024, the test is no longer offered directly to patients. This decision was influenced by communications with the Alzheimer’s Association and other biomarker researchers according to MedPage Today.
The blood test measures the Aβ42/40 ratio in the blood, with a ten-day turnaround time to get the results.
How does the test work?
A doctor can order the test online and set up an appointment at a Quest Diagnostics laboratory.
How fast are results available?
The results are available within ten business days after blood is drawn
How much does the test cost?
The test costs $400 and is not currently covered by Medicare or other insurers.
How accurate is the test?
Comparing this test to the others that are available on the market is tricky. The other tests are ordered by doctors when they suspect a patient might have Alzheimer’s due to their cognitive symptoms. That means less people take those tests, and the ones that do have a higher likelihood of having Alzheimer’s anyways. But this test can be ordered by almost anyone. That means that more people without cognitive symptoms will take the test and potentially receive incorrect results. Neurologists have expressed hesitation about going this route.
What does that mean for you?
If someone has amyloid plaques in the brain, the test will provide a positive result 89 percent of the time. The test has been criticized because of its very high rate of false positives: 29 percent of people who take the test will receive a false positive. This isn’t as much of a problem if the test is ordered by doctors for symptomatic patients, but when almost anyone can take the test, it means many more people could be exposed to a false positive result. Even in a best-case scenario, there will be more false positives than actual people with the disease that are tested.
Dr. David Weisman, a neurologist at Abington Neurological Associates, said this means that the test “totally sucks” for screening purposes.

ALZpath’s ALZpath Dx test
In March 2024, ALZpath launched the ALZpath Dx test, which measures the levels of pTau-217. In a study of 786 patients, the blood test was able to detect beta-amyloid and tau pathology as well as a cerebrospinal fluid test or amyloid PET scan.
How does the test work?
A neurologist will order the test and set up a blood draw which is then sent off to a lab for processing. Right now, the processing of the test is done at Neurocode Laboratory but it may become available at other laboratory providers in the future.
How fast are results available?
The results are available within ten business days after blood is drawn
How much does the test cost?
A spokesperson for ALZpath said that the test will cost between $300 to $500. It is currently not covered by Medicare or other insurers.
How accurate is the test?
Based on ALZpath’s studies, the test correctly diagnoses someone with Alzheimer’s disease 95 percent of the time. The chances of receiving a false positive result are 5 percent.
Michelle Mielke, a professor at Wake Forest School of Medicine, who was not involved in the development of these tests, told Being Patient that these tests have good clinical accuracy as a diagnostic marker of amyloid in the brain for people with mild cognitive impairment. “Additional real-world studies will be needed to determine accuracy in older adults with multiple chronic conditions and cognitive impairment,” she added.

Eli Lilly’s CertuitAD
Drugmaker Eli Lilly has also developed a blood test that is now available for order by clinicians. The test measures the levels of pTau-217 in the blood.
The company tested the accuracy of the test in 2,071 participants over the age of 60 with cognitive decline who didn’t have other medical conditions, like high blood pressure or diabetes. Eli Lilly intends to present these results at a conference and publish them in a scientific journal.
How does the test work?
A neurologist will order the test and set up a blood draw which is then sent off to a lab for processing.
How fast are results available?
The results are available within ten business days after blood is drawn
How much does the test cost?
The test costs $195. It is currently not covered by Medicare or other insurers.
How accurate is the test?
The test correctly detected amyloid pathology in the brain 91 percent of the time and returned a false positive result 10 percent of the time.
UPDATED – APRIL 25, 12:40 PM EST: Added information about Eli Lilly’s CertuitAD blood test.
UPDATED – APRIL 2, 1:31 PM EST: Added information about the ALZpath Dx and Labcorp pTau-217 blood test which were released after the initial publication of the article. As of March, Quest Diagnostic’s test is also no longer offered direct to patients and must now be ordered through a doctor.