It seems like, following the FDA's controversial July 2021 approval of Biogen's Alzheimer's drug Aduhelm, everyone is mad at someone. Here are the main reasons why.
UPDATE: 3 March 2024, 8:18 P.M. ET. In February 2024, Biogen took Aduhelm off the market, citing financial concerns. Although the drug did receive accelerated, conditional FDA approval for the treatment of early Alzheimer’s disease in 2021, it is no longer available to new patients. The company announced it would sunset trials in May 2024 and cease supplying the drug to current patients in November 2024.
Download this explainer as a PDF.
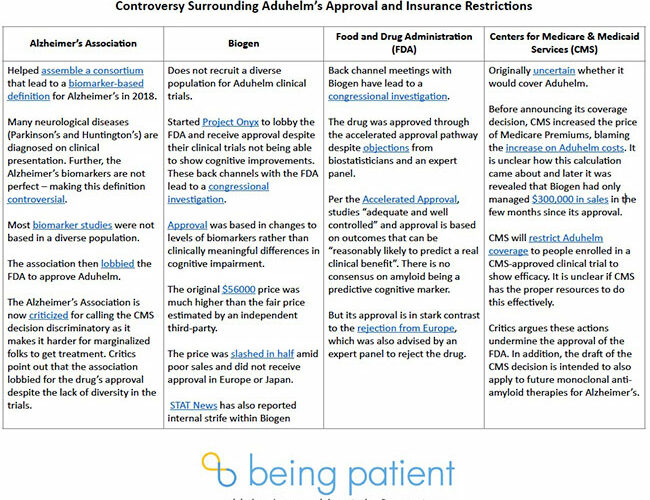
The Alzheimer’s Association:
- Helped assemble a 2018 consortium that led to a biomarker-based definition of Alzheimer’s, controversial because most neurological diseases are still diagnosed through symptoms.
- Lobbied the FDA to approve Aduhelm, even though the trials did not reliably show that the drug reduced cognitive impairment.
- Called the CMS decision discriminatory because it limits access for marginalized groups. Critics say this feels contradictory, considering the organization lobbied for Aduhelm’s approval despite that its clinical trials lacked racial diversity.
Aduhelm’s drugmaker, Biogen:
- Has not included a diverse population in Aduhelm clinical trials even though Hispanic and African American populations are at a higher risk. It is also unclear whether the drug works similarly in these populations.
- Started Project Onyx to lobby the FDA and receive approval for Aduhelm despite cloudy efficacy data.
- Had back-channel meetings with the FDA that have triggered a congressional investigation.
- Initially marketed Adulem at $56,000 — much higher than the fair price estimated by an independent third party.
- Slashed the drug’s price in half amid poor sales and rejection by regulating agencies in Europe and Japan.
- Internal strife at the company following Aduhelm’s approval.
The Food and Drug Administration:
- Originally approved Aduhelm as a broad-label treatment even though it had been tested in mild Alzheimer’s. The FDA later walked back the broad-label.
- Had back-channel meetings with Biogen that have triggered a congressional investigation after the backlash from the public, scientists and even the-then chief of the FDA, Janet Woodcock.
- Approved Aduhelm with accelerated approval despite objections from biostatisticians and an expert panel.
- Three members of the advisory panel resigned in protest of the approval.
- Approval was based on biomarkers rather than differences in cognitive impairment. For drugs passing through the accelerated approval pathway, this is allowed as long as these outcomes can predict a real clinical benefit. However, it is debatable whether clearing amyloid actually slows the rate of cognitive decline.
- Contradicted in its Aduhelm approval decision by subsequent rejections from European and Japanese regulators.
CMS:
- Initially waffled about whether it would cover Aduhelm, leaving patients and families in limbo.
- Increased the price of Medicare premiums for all subscribers — blaming Aduhelm costs, despite very low Aduhelm sales — before ultimately announcing its decision to dramatically limit coverage of the drug.
- Announced plans to only cover Aduhelm for people enrolled in a CMS-approved clinical trial of the drug’s efficacy, despite that Biogen is already running such a trial.
- Undermined the authority of the FDA in announcing its own make-or-break vetting process for Aduhelm and all other Alzheimer’s monoclonal anti-amyloid therapies to follow.



Living with someone that has and needs help everyday – any opportunity for families to have and receive new meds should not have the quality of their lives dictated by what and how it is available. My spouse may never get a chance then to slow down a horrible disease. Thank you paula
I would like a list of all meds applicable to slow the progression of alzheimers symptoms and their availability and cost. Would also like all articles supporting items on the list.
Biased article which fails to mention that Aduhelm removes amyloid plaque and tau tangles. In reality symptomatic improvement is extremely hard to detect with any accuracy.