Doctors struggle to predict who with Alzheimer’s pathology will develop symptoms. Two new cerebrospinal fluid biomarkers could help change that.
Everyone with Alzheimer’s disease has beta-amyloid plaques and tau tangles in their brain. But despite advances across blood tests, cerebrospinal fluid tests, and brain imaging, doctors still can’t tell for certain whether cognitively healthy individuals who test positive for these biomarkers will ever end up actually developing Alzheimer’s cognitive symptoms.
A new study published in Nature Medicine zeroed in on two predictive proteins in the cerebrospinal fluid that foretell cognitive decline and predict who will progress from mild cognitive impairment to Alzheimer’s disease.
“The field needs new biomarkers to predict who will actually get dementia from the subset of the population that has the pathology,” said Hamilton Oh, a postdoctoral researcher at the Icahn School of Medicine at Mount Sinai, who led the study. “We need to know who’s going to get dementia to know who to treat.”
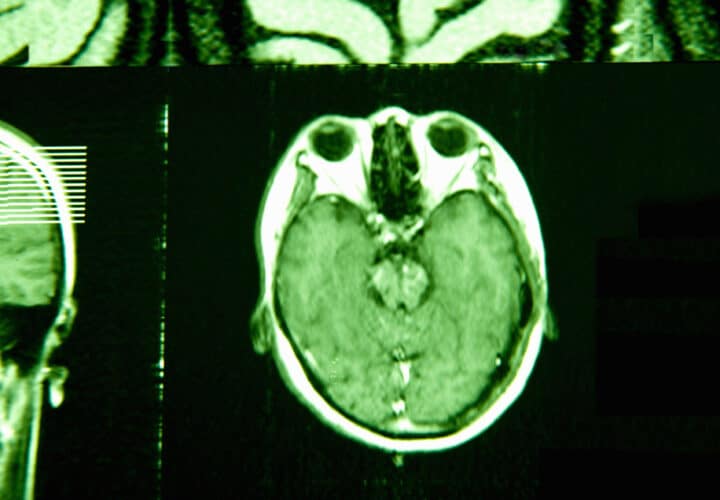
Researchers analyzed the cerebrospinal fluid of 3,397 individuals across six different cohorts, which included participants with both rare and common forms of Alzheimer’s disease, as well as healthy controls. They used a test that allowed them to measure the levels of thousands of different proteins at once, and searched for elusive proteins that could predict cognitive decline.
Two proteins came out on top. Higher levels of YWHAG protein and lower levels of neuronal pentraxin 2 (NPTX2) were linked to cognitive impairment. Both proteins are involved in regulating the synapse, where two neurons meet and communicate with each other. In cohorts of people with genetic forms of Alzheimer’s disease, the ratio of these proteins increases before amyloid plaques and tau tangles appear.
While high levels of beta-amyloid and tau biomarkers make a cognitively healthy person four times as likely to develop cognitive impairment in their lifetime, people with a high YWHAG/NPTX2 ratio are 15 times as likely. The ratio of these proteins also gave a very accurate prediction as to whether someone with mild cognitive impairment and amyloid and tau would progress to early-stage symptomatic Alzheimer’s.
The team also explored whether these proteins might work as blood-based biomarkers. But since many of the study participants were enrolled before Alzheimer’s blood tests were widely available, the researchers didn’t have enough blood samples to confirm their potential. Future studies will be needed to get to the bottom of that question.
Since most of the participants included in these Alzheimer’s cohorts are white and of European descent, it’s not clear how ethnicity might be influencing these biomarkers. Other blood and brain imaging biomarkers differ between Black and white Americans, making standard Alzheimer’s tests less reliable.
“The major next step is to see whether these two proteins actually change in other forms of dementia,” Oh told Being Patient. “It’s possible that they’re not Alzheimer’s specific.”
Integrating YWHAG/NPTX2 into clinical practice and trials
Nicholas Ashton, the senior director of the fluid biomarker program at Banner Health, who wasn’t involved in the study, told Being Patient that the results were “really robust” and relevant to clinical practice.
Understanding who will decline is important on a personal level for patients. “They want to know, just like we do in other types of areas of medicine, ‘What’s my prognosis?’” said Ashton. If these biomarkers are integrated into practice, they could inform patients whether they have another two or ten years of living independently before substantial cognitive impairment.
Oh said that the levels of these proteins could be measured in future clinical trials and could help determine which patients will benefit most from anti-amyloid drugs. Leqembi and Kisunla are approved in the U.S. for treating early Alzheimer’s disease, which includes patients with mild cognitive impairment who have amyloid and tau biomarkers.
But clinicians have no way of knowing whether they’re prescribing the drug to someone who is likely to progress to the next stage soon. Based on the levels of YWHAG/NPTX2, “you might actually be relatively fine for a long time, in which case, maybe it’s not worth the risk” to take these drugs, said Oh.
These biomarkers could also improve the way scientists study drugs in clinical trials. Currently, trials measure amyloid, tau, and other biomarkers in conjunction with cognitive assessments. However, this isn’t enough to tell whether a drug is slowing the progression of the disease.
According to Ashton, YWHAG/NPTX2 “would be a better, more objective indicator that we’ve affected the disease course rather than a quite vague clinical assessment.”
The researchers were supported by multiple grants from the National Institutes of Health (1K99AG088304-01, R01AG044546, P01AG003991, RF1AG053303, RF1AG058501, U01AG058922, P30AG066444).



I would like to keep up with research and anything that might give me hope. I am 76 and I was diagnosed in January 2025. I am emotionally reactive…I cry and get afraid of getting closer to the end of my life. I can take care of myself except my son works with finances.
Hi Patricia, thank you for sharing your expirence. You can check out our Q1 Trials Update for the latest information on Alzheimer’s research here: https://www.beingpatient.com/trials-update-2025-q1/ and feel free to sign up for our quarterly Trials Update newsletter here: https://www.beingpatient.com/bp-trials-updates/?utm_source=organic&utm_medium=social.
Thank you for keeping me up with any new
ways to add time to my life and I would like to be a part of any research and trials.