Cassava Sciences' experimental Alzheimer's drug simufilam is under fire again. City University of New York filed an investigative report to explore allegations of misconduct by scientists who developed simufilam, finding the data to be "highly questionable." As of Oct. 27, investigation itself is now under investigation.
DEVELOPING STORY, updated 30 October, 2023: In August 2021, the biotechnology company Cassava Sciences — makers of simufilam, an experimental Alzheimer drug currently in Phase 3 clinical trials — was accused of scientific misconduct and fraud by a shareholders’ rights law firm. Cassava Sciences’ stock took a tumble after the firm raised concerns about data on the drug via a Food and Drug Administration citizen petition (a public request made by individuals and community organizations to change or alter a health policy). The FDA citizen petition suggested Cassava should suspend simufilam’s clinical trials until new trial data is reviewed and audited. It’s been drama ever since.
While Cassava Sciences experimental Alzheimer’s drug simufilam proceeds through Phase 3 clinical trials, investigations into the company and the drug data are currently underway by the U.S. Justice Department and the U.S. Securities and Exchange Commission. In response, the company has lobbed lawsuits at scientists and investors who have publicly criticized the simufilam data in question.
According to David Vaux, deputy director of scientific integrity and ethics at the Walter and Eliza Hall Institute of Medical Research, the concerns brought up in the 2021 FDA citizen petition were legitimate and troubling. Eventually, the citizen’s petition was denied on a technicality.
But now, an internal report from an investigative committee at the City University of New York (CUNY), leaked to Science Magazine by an unknown source, has accused CUNY faculty member Hoau-Yan Wang — a neuroscientist who worked with Cassava on the development of simufilam — of scientific misconduct.
The 50-page report by this investigative committee — a four-member panel of researchers at the university — calls into question 20 research papers, many of which informed Cassava’s approach to developing simufilam, according to Science.
Another wave of allegations of misconduct and manipulated data
Five months ago, the CUNY committee filed an investigative report into Wang’s research and shared their findings with CUNY’s Research Integrity and Compliance Office.
The report investigated 31 allegations of scientific misconduct made by researchers as well as the FDA citizen petition. Under particular scrutiny were several figures from scientific papers generated by Wang which appeared to show characteristic signs of manipulation in the form of data irregularities known as western blots.
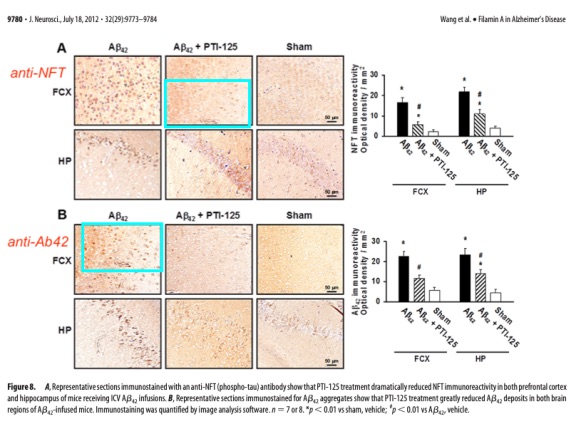
Wang did not cooperate with the investigation and the university has yet to take any public action in response to the findings. Rather, he was unable or unwilling to provide any original data related to his work on Cassava Sciences’ candidate Alzheimer’s drug.
“It appears likely that no primary data and no research notebooks pertaining to the 31 allegations exist,” the report authors wrote. “Dr. Wang’s failure to store original data and maintain research records is reckless and clearly not in keeping with the standard practices in the field. It is our conclusion that this amounts to significant research misconduct.”
The CUNY report also placed blame on Lindsay Burns, Cassava’s senior vice president of neuroscience, who co-authored several of the 20 papers.
According to the report, Wang was unwilling or unable to provide any of the raw data or notebooks. In some cases, the investigators found, protocols for experiments were written down on scrap paper and thrown away afterward. Wang also failed to prioritize the storage of raw data. These missteps are consistent with the U.S. HHS’s Office of Research Integrity’s definition of academic misconduct.
A scientific integrity expert, Elisabeth Bik, PhD, told Being Patient that CUNY and the National Institute of Health which funded these studies require the notebooks and data to be saved for at least six years.
“About half of the publications investigated in the report are less than six years old, and there should have been originals to share,” she said. “It is very concerning that Dr. Wang threw out most of his primary data.”
Ultimately, the report states, there was“long-standing and egregious misconduct in data management and record keeping by Dr. Wang.” As for the manipulated data, investigators wrote that they could not objectively determine whether data was manipulated, due to Wang’s lack of record-keeping.
Cassava’s response
Representatives for Cassava Sciences interpreted the CUNY report differently. A Cassava press release suggested that this report’s leak to Science was another act in a long-standing campaign to devalue the company’s stock. The press release also pointed out that “CUNY’s report “makes no findings of data manipulation.” (Indeed, the CUNY report authors state they were unable to conclusively, objectively determine data manipulation on account of the lack of original data provided to them: “Dr. Wang’s failure to store original data and maintain research records is reckless and clearly not in keeping with standard practices in the field,” the report authors noted.)
The Cassava release also alleges that CUNY investigators are not objectively able to assess allegations of misconduct through no fault of Wang’s, but rather to the university’s own record-keeping failures. (During the investigation, Wang pointed the finger at CUNY’s IT department to explain some of the missing data.)
Cassava president and CEO Remi Barier reiterated this stance to Being Patient in an email: “I urge you to ask the hard questions: Who, why and when was [the CUNY report] leaked, and did the report find what it was looking for, i.e., evidence of data manipulation?”
Barbier’s stance echoes his 2021 statement in response to the initial wave of accusations about falsified or doctored data: Back then, he said the allegations against Cassava were flat-out false and implied that the group behind the complaints was motivated by a desire to short Cassava’s stock.
In an email to Being Patient in 2021, he denied all allegations of problematic research, and said of the original FDA petition that the firm’s claims were surprising and unfounded: “Cassava Sciences stands behind its science, its scientists and its scientific collaborators, and is responding to ensure the facts are known and respected. As a science company, we champion facts that can be evaluated and verified. This helps people make informed choices,” he wrote at the time.
What does the CUNY report mean for simufilam and its Phase 3 clinical trials?
Wang’s lab was instrumental for developing simufilam, discovering that a protein called filamin-A was misfolded in Alzheimer’s disease. Simufilam is supposed to fix the misfolded protein, leading to cognitive improvement.
However, the studies supporting the role of filamin-A misfolding in Alzheimer’s, and simufilam’s ability to fix the protein, are mainly published by the company, its collaborators, and other work funded by Cassava. More research is needed to prove out this approach’s impact on Alzheimer’s pathology in the brain.
The drug is currently in its Phase 3 clinical trial which has now recruited more than 800 volunteers to test its treatment for mild-to-moderate Alzheimer’s disease.
The yet unpublished, leaked CUNY report has renewed calls to halt clinical trials.
According to Ivan Oransky, co-founder of Retraction Watch which tracks cases of scientific misconduct and retractions of published scientific research papers, it may take several months or years for the university to make an official decision.
“These cases are shrouded in secrecy,” Oransky told Being Patient. “They [institutions] want to be able to deliberate at their pace and are worried discussing these issues too much would erode trust in science. But it’s actually the act of not talking about it that erodes trust.”
CUNY’s Research Integrity and Compliance Office did not respond to Being Patient’s request for comment. Since the report leaked, however, news organizations that covered the report have been under fire as has the university itself, lobbing allegations that the investigation is corrupt. Three days after this article was originally published, CUNY released a statement.
“CUNY is committed to ensuring that its investigative processes are held to the highest procedural and ethical standards and that the fairness of the proceedings is preserved for all parties,” the press statement read. “To that end, any finding regarding allegations of research misconduct must be reliable and credible. Because questions regarding the confidentiality and integrity of this investigation have been raised, CUNY will stay the underlying inquiry into the allegations regarding Dr. Wang’s research until such time as the University completes a comprehensive investigation of the process.”
In other words, the university must first investigate the investigation. If the allegations of investigative misconduct against CUNY are cleared, the department to whom the internal report (which was later leaked to SCIENCE magazine) was submitted can investigate the findings of the original four-person investigative committee.



I am very concerned about the funding for being patient and printing an article like this. Please see the poster at the CTAD conference supporting the mechanism of action. CUNY’s own policy states records need to be maintained for 7 yrs, but I don’t see this mentioned in the 50 page leaked report. The report has ties to short sellers – very skeptical all around. Wish you could secure funding from sources than don’t influence your reports. Very unfortunate for the Alzheimers community that trusts your reports.
Hi Nate, We are very concerned about readers posting baseless allegations about our funding sources in our comments. 🙂 This isn’t an op-ed full of wild opinions — it’s a straightforward news rundown of the Cassava investigation to date. Our reporter has dug deep on this story in the past and verified the details of his reporting with multiple sources — and we stand by it. To address your question about funding head on, we launched on out-of-pocket funding from the editor in chief, whose mother lives with Alzheimer’s disease, earned from her career in journalism. Thanks to community support and support from readers, we’re now able to be primarily funded by ad revenue from display ads, and from a limited number of clearly disclosed content partnerships in which we retain complete independence and editorial control over the content we produce to our publication’s journalistic standards. Of course, we’d have to wonder about your undisclosed motivations here. That said, as science writers, we always appreciate skepticism, and if this is indeed a scandal within a scandal that has yet to be discovered by the media, and you have concrete evidence of conflicts of interest by the parties who conducted the investigation — or even a halfway credible lead — our managing editor is standing by for your email, and she will look forward to directing it as appropriate so that our small, grassroots but dedicated reporting team can investigate.