From long-awaited trial results to new drug developments, here are key updates from Alzheimer’s and dementia clinical research between January and April — including highlights from the AD/PD 2025 conference.
This installment of our quarterly Trials Update captures the latest developments in Alzheimer’s and other dementia clinical trials, from January 2025 to present. We’ve included all the latest news, including our reporting on early findings from the AD/PD 2025 conference in Vienna, where researchers shared updates on vaccine trials, small molecules, stem cell therapies, and more.
On the disease modification front, several pills for Alzheimer’s treatment are still in the race — or falling out of it. One drug targeting ApoE4 carriers failed to meet its goal, while others, including semaglutide and buntanetap, are pressing forward in large Phase 3 trials. Biologics are gaining ground: Antibody drugs like Leqembi are being tested for prevention, and several vaccine trials are underway — including in people with Down syndrome.
When it comes to treating symptoms, multiple drugs and devices are being tested to help manage Alzheimer’s symptoms — from memory loss to agitation. Trials are underway for schizophrenia and sleep drugs repurposed to address psychiatric and cognitive symptoms, as well as experimental headsets and brain stimulation therapies. Read on below for all the details.
And right now, 88 different clinical trials for Alzheimer’s are recruiting. (We’ve pulled them together for Trials Update subscribers in an interactive, browsable list. Not yet signed up for the quarterly Trials Update? Subscribe for free, here.)
Disease-modifying drugs
Small-molecule drugs
Some Alzheimer’s pills have dropped out of the race. Others are nearing the finish line with Phase 3 trials:
ALZ-801/valiltramiprosate
- Industry sponsored?: Alzheon
- Latest trial phase: Failed to meet primary goal in Phase 3 trial
- Next steps: Company is in discussions with regulators but has not decided on next steps.
- New updates: Yes
Alzheon’s pill, ALZ-801, designed to prevent beta-amyloid plaque formation, failed to slow cognitive decline in people with early Alzheimer’s and two copies of ApoE4. Some modest benefits were observed in participants with MCI, but the trial was not designed to confirm efficacy in this subgroup. The company is in talks with regulators and weighing next steps.
Alzheon Alzheimer’s Pill Misses the Mark in Phase 3 Trial, Hints at Benefit in MCI
AR1001
- Industry sponsored?: AriBio
- Latest trial phase: Ongoing Phase 3 trial.
- Next steps: Present some Phase 3 data in 2025.
- New updates: No
Originally developed for erectile dysfunction, AR1001 is now in a 1,150-person Phase 3 trial for early Alzheimer’s. Initial results are expected later this year, with final results projected by 2027.
ASN51
- Industry sponsored?: Asceneuron
- Latest trial phase: Terminated Phase 2 trial.
- Next steps: Unknown.
- New updates: Yes
Asceneuron halted its tau-targeting drug trial early, citing a strategic pivot. No further details on development plans have been announced.
Blarcamesine
- Industry sponsored?: Anavex Life Sciences
- Latest trial phase: Completed Phase 2/3 trial.
- Next steps: Submitted drug for approval in Europe.
- New updates: No
At the end of November 2024, Anavex submitted its sigma-1 targeting drug for approval in Europe, despite high dropout rates and criticisms of its efficacy claims. The company is facing shareholder lawsuits alleging trial data misrepresentation.
Butanetap
- Industry sponsored?: Annovis Bio
- Latest trial phase: Initiated Phase 3 trial.
- Next steps: Results of the trial are expected in 2028.
- New updates: Yes
Annovis Bio reported disappointing results on their first attempt at a Phase 3 trial for their experimental disease-modifying Alzheimer’s pill buntanetap, but they aren’t throwing in the towel just yet: They saw an indication that there might be a positive effect for one subgroup of those trial participants so in February, they launched a new 18-month, 750-person Phase 3 trial. Results are expected in 2028.
Semaglutide
- Industry sponsored?: Novo Nordisk
- Latest trial phase: Ongoing Phase 3 trial.
- Next steps: Results of the trial are expected this year.
- New updates: Yes
Some early studies suggested a class of diabetes drugs called GLP-1 agonists — which have been proven to also help people lose weight and reduce the risk of heart attacks or stroke — might protect against Parkinson’s, Alzheimer’s and other forms of dementia too. Now, Novo Nordisk is testing semaglutide (Ozempic, Wegovy) in two large Phase 3 trials (each with 1,840 participants) for early Alzheimer’s. Results are expected by the end of 2025.
Simufilam
- Industry sponsored?: Cassava Sciences
- Latest trial phase: Discontinued
- Next steps: The company will pivot the drug to test it in a genetic seizure disorder.
- New updates: Yes
After failing to show efficacy in two Phase 3 trials, Cassava announced in March that it would end its Alzheimer’s program. The company is facing lawsuits and a DOJ fraud indictment. It now plans to pivot to test its drug simufilam in a rare epilepsy disorder caused by a genetic disease called tuberous sclerosis complex.
Biologics
Biologics are gaining traction, from antibody drugs aiming to prevent Alzheimer’s to vaccines and cell therapies still in early testing.
ACI-24.060
- Industry sponsored?: AC Immune SA and Takeda
- Latest trial phase: Phase 1a/2
- Next steps: Trial expected to finish in 2026.
- New updates: Yes
AC Immune is testing its beta-amyloid vaccine for Alzheimer’s prevention in cognitively healthy individuals at risk and people with Down syndrome. The trial is ongoing through 2026.
ALZN-002
- Industry sponsored?: Alzamend Neuro
- Latest trial phase: Phase 1a/2
- Next steps: Trial expected to finish in 2028.
- New updates: Yes
Alzamend Neuro is running a 30-person Phase 1-2 trial to evaluate this beta-amyloid vaccine for mild to moderate Alzheimer’s. Results are expected in 2028.
GV1001
- Industry sponsored?: GemVax/Kael Bio
- Latest trial phase: Phase 3
- Next steps: Trial expected to finish in 2028.
- New updates: Yes
Originally developed as a cancer vaccine, GV1001 is now being tested for moderate-to-severe Alzheimer’s in a Phase 3 trial in South Korea. The trial is expected to finish in 2028.
JNJ-2056
- Industry sponsored?: AC Immune SA and Jannsen
- Latest trial phase: Phase 2b.
- Next steps: Trial is currently being planned.
- New updates: Yes
At the AD/PD 2025 conference, AC Immune SA and Jannsen announced that a tau vaccine, JNJ-2056, for Alzheimer’s prevention is in the planning stages. Their forthcoming Phase 2b trial is not yet registered on ClinicalTrials.gov.
Leqembi
- Industry sponsored?: Eisai and Biogen
- Latest trial phase: Approved by the FDA
- Next steps: Injectable version of the drug and two additional ongoing studies.
- New updates: Yes
In trials:
- The drug is being tested in the AHEAD 3-45 study to see if it could prevent Alzheimer’s disease in asymptomatic individuals with risk factors for the disease. Results are expected in 2028.
- The drug is also being tested in conjunction with an anti-tau antibody in a Phase 2 trial. The results are expected in 2027.
On the market:
- The FDA will decide by August whether or not to approve an injectable version of the drug, which could be delivered at home.
- A lower dosage of Leqembi was greenlit in January, approved for people who have been taking it for more than 18 months.
- EU regulators reversed an earlier rejection of Leqembi, approving it in the EU. The drug will be restricted to people who don’t have two copies of the ApoE4 gene to minimize risk.
Laromestrocel
- Industry sponsored?: Longeveron
- Latest trial phase: Completed Phase 2 trial.
- Next steps: No Phase 3 trial announced yet.
- New updates: Yes
Longeveron published data from a 49-person Phase 2 trial into its “stem cell” therapy for Alzheimer’s. While results showed safety, independent experts expressed skepticism about efficacy. The company has been offering the unapproved at cost in the Bahamas. No Phase 3 trial has been announced.
Stem Cells for Alzheimer’s? Neurologists Weigh In on Longeveron’s New Data
Kisunla
- Industry sponsored?: Eli Lilly
- Latest trial phase: Approved by the FDA
- Next steps: Multiple ongoing late-stage trials to test the drug in other populations and doses.
- New updates: Yes
In March, EU regulators rejected approval of Kisunla over ARIA concerns. Trials continue in non-American populations (results expected in 2027), investigating different dosing regimes that may reduce the risk of side effects. Those results are being presented at various conferences this year. There are also plans in place to test Kisunla as an Alzheimer’s prevention in people with Down syndrome, with results expected in 2027.
Remternetug
- Industry sponsored?: Eli Lilly
- Latest trial phase: Phase 3.
- Next steps: The company has filed an application to approve an injectable version of the drug.
- New updates: No
Four Phase 2/3 trials are underway to assess this antibody’s effects on early Alzheimer’s and prevention in people with genetic risk. First results are expected in 2026.
Troculeucel (formerly known as SNK01)
- Industry sponsored?: NK Gen Biotech
- Latest trial phase: Completed Phase 1/2 trial. Phase 2 trial ongoing.
- Next steps: Completion of Phase 2 trial in 2025.
- New updates: Yes
Derived from patients’ own “natural killer” immune cells, infusion-based drug toculeucel is being tested for moderate Alzheimer’s. Following positive data from three participants in a Phase 1 trial (presented at the AD/PD conference in April), the company launched a 36-person Phase 2 trial, expected to conclude at the end of 2025.
Remternetug: Alzheimer’s Prevention Trial Enrolls People as Young as 18
Trontinemab
- Industry sponsored?: Roche
- Latest trial phase: Completed Phase 1/2 trial.
- Next steps: The company is planning a Phase 3 trial.
- New updates: Yes
Like a Trojan Horse, Roche’s anti-amyloid drug trontinemab “tricks” the blood-brain barrier to get into the brain more easily. As a result, it is administered at lower doses and clears beta-amyloid plaques faster. The company presented promising data from its 114-person phase 1/2 trial in April. The overall rates of ARIA are substantially lower than what is seen with Leqembi and Kisunla, and if these results hold up in a larger Phase 3 trial, it may be substantially safer.
Symptomatic treatments
Several drugs and devices are being tested to help manage Alzheimer’s symptoms — from memory loss to agitation.
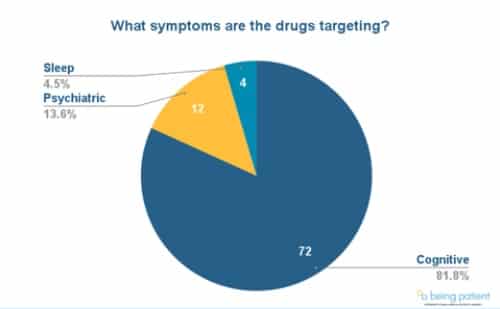
For memory symptoms
Piromelatine
- Industry sponsored?: Neurim Pharmaceuticals Ltd
- Latest trial phase: Ongoing Phase 2/3 trial.
- Next steps: Results expected in 2025
- New updates: No
Originally developed for sleep disorders, piromelatine may offer cognitive benefits to certain people with mild Alzheimer’s. Results from Neurim Pharmaceuticals’ Phase 2/3 trial are expected this year.
Rotigotine and rivastigmine
- Industry sponsored?: No
- Latest trial phase: Phase 3 trial ongoing.
- Next steps: Results expected in 2025.
- New updates: No
An ongoing 348-person trial in Italy is exploring whether combining rotigotine (a Parkinson’s drug that affects the levels of a brain signaling molecule called dopamine) with an already-approved cholinesterase drug can improve cognition in people with mild to moderate Alzheimer’s. The trial is expected to finish this year.
For psychiatric symptoms
AXS-05
- Industry sponsored?: Axsome Therapeutics
- Latest trial phase: Completed two Phase 3 trials
- Next steps: Filing for FDA approval later this year
- New updates: No
Approved by the FDA in 2022 for depression under the brand name Auvelity, Axsome’s AXS-05 is being tested to help treat Alzheimer’s-related agitation. The company shared data from two Phase 2-3 trials in 2020 and 2022. Now, after yet two more Phase 3 trials, the company plans to seek FDA approval, though some experts are saying the trial results indicate that the drug isn’t effective.
IGC AD-1
- Industry sponsored?: IGC Pharma
- Latest trial phase: Ongoing Phase 2 trial
- Next steps: Completion of Phase 2 trial
- New updates: No
In a 164-person Phase 2 trial, this oral, cannabinoid-based agitation drug showed slight improvements at six weeks, but more research is needed to determine efficacy. The trial concludes in June 2025.
KarXT
- Industry sponsored?: Karuna Therapeutics
- Latest trial phase: Two Phase 3 trials
- Next steps: Completion of the first Phase 3 trial later this year
- New updates: Yes
Last year, KarXT, became the first new drug approved for treating schizophrenia in the US since the 1950s. Karuna Therapeutics is testing the drug in two ongoing Phase 3 trials for Alzheimer’s-related psychosis. Results from the trials (with 380 and 400 participants respectively) are expected in 2025 and 2026.
Medical devices
It’s not yet clear whether these could be disease-modifying — but early results from new technologies are turning heads.
Repetitive transcranial magnetic stimulation (rTMS)
- Industry sponsored?: Sinaptica Therapeutics
- Latest trial phase: Completed Phase 2 trial.
- Next steps: Phase 3 trial.
- New updates: Yes
Sinaptica is using a magnet to zap a specific part of the brain which could help slow Alzheimer’s disease progression. In a 48-participant Phase 2 trial, Sinaptica’s rTMS protocol slowed cognitive decline over the course of a year by just over 1 point on an 18-point scale. Details of an upcoming Phase 3 trial have not yet been announced.
SPECTRIS
- Industry sponsored?: Cognito Therapeutics
- Latest trial phase: Ongoing Phase 3 trial
- Next steps: Completion of Phase 3 trial in 2026.
- New updates: Yes
Last year, Cognito announced that its brain-stimulating light-and-sound headset, Spectris, showed lasting effects on brain volume and daily functioning in a preliminary study. Safety and efficacy results are expected from the ongoing 670-person Phase 3 trial by mid-2026.




As a Memory Care Specialist and Geriatrician… I thank you for this.
Hi Karen, thank you for being a part of our community. We appreciate your support!
Being Patient is making a huge difference forever for those who have these terrible diseases.
Hi Eaton, thank you for being a part of our community!
Thanks for this wonderful synopsis of current events in Alzheimer’s research. Although, the developments have been largely disappointing, some recent trials appear to be showing some promising results.
Hi Joe and Trish, thank you for being part of our community. Sign up for our quarterly Trials Update newsletter to stay up to date on the latest trial news: https://www.beingpatient.com/bp-trials-updates/?utm_source=organic&utm_medium=social – take care.