The latest news about Biogen’s controversial Alzheimer’s drug popped into my email at 8:26 a.m. last Wednesday. Coincidentally it arrived just as I was trying to pull myself out of bed to get ready to leave for an infusion of that very same controversial Alzheimer’s drug.
The email from Biogen’s corporate communications team was full of scientific jargon but the bottom line was clear: Biogen and its corporate partners had finally sent an application for approval to the U.S. Food and Drug Administration for aducanumab, a drug that if approved “would become the first therapy to reduce the clinical decline of Alzheimer’s disease.”
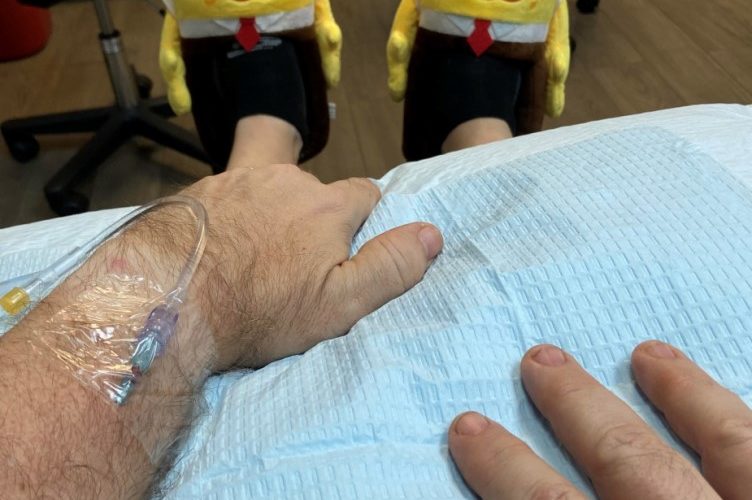
I learned of my diagnosis of early onset Alzheimer’s four years ago after applying to join the aducanumab trial. Month after month, I offered up my left arm for an IV hookup and watched as drop-by-drop a bag of clear liquid emptied into my vein.
In the Spring of 2019, I was among the thousands of patients left reeling when Biogen announced that it was ending the aducanumab trials because the drug had failed a “futility analysis.” And as a reporter for Being Patient, I was among the first to write about Biogen’s shocking reversal last October.
I even sat in a packed hotel conference room in San Diego as 1,600 doctors, researchers and financial analysts listened — many with great skepticism — as a Biogen scientist presented the data that led to the reversal. It was at that conference that Biogen promised to seek FDA approval for aducanumab in early 2020.
But that deadline kept slipping and the most recent news from Biogen was that an application would be filed sometime after September 2020. So yes, early July was definitely a surprise.
Biogen said the application involved approximately 4,500 files of information, a total of 2.5 million pages of what I’m sure was an easy-to-read narrative. Just kidding. After listening to the Biogen presentation in San Diego, I’m quite sure those 2.5 million pages were filled with utterly incomprehensible discussion of statistical data points.
While absolutely critical, those data points mean less to trial patients than what Dr. Steven Salloway, the Director of the Butler Hospital Memory and Aging Program at Brown University, said in the Biogen news release.
“For many people living with the early stages of Alzheimer’s disease, maintaining independence for as long as possible is the ultimate goal,” Salloway said. “If we can help slow the progression from one stage to the next, this could preserve independence, which, in turn, could have truly meaningful benefits for people living with the disease and their loved ones.”
“Aducanumab,” he added, “represents a potential breakthrough that we hope will provide a treatment foothold in the fight against Alzheimer’s disease.”
James Kupiec, Chief Medical Officer at ProMIS Neurosciences, told Being Patient that he agreed the Biogen application represented a “landmark” but said that “its real value is its effect on research and development for next-generation therapies.”
Anticipating that aducanumab will win approval in 2021, Kupiec added that “aducanumab is good but, perhaps more importantly, its advancement validates the science driving next-generation versions, which will be better.”
It is that science that drives me. While I’m hopeful that aducanumab is slowing the progression of Alzheimer’s in me, I’ve long said that I don’t really believe that science will advance quickly enough to save me.
I continue to hold that belief even as I participate in a new trial of aducanumab that Biogen and Eisai launched while waiting for the FDA’s decision. The new trial is open to the 4,000 or so people who participated in the original trials.
Instead I participate in research trials for future generations, for my nieces and nephews and their children. And in my own little way, I try to encourage others to participate in trials.
Without people willing to participate in trials, test medications cannot proceed. The New York Times reported a few years ago that the struggle to find trial participants is becoming debilitating.
With that in mind, I launched my “silly shoe challenge” and each time I get an infusion or MRI I wear a different pair of unusual shoes. My collection has ranged from SpongeBob slippers to thigh-high-red-stiletto kinky boots. Last week, I wore a pair of Freudian slippers.
My theme is that you can do good — participating in research trials — while still having fun. And while trials do have their challenging moments, I’ve met some incredible people who care deeply about finding a treatment or cure for Alzheimer’s.
Getting that email from Biogen on the morning of my second infusion in the new aducanumab trial made all the needle pokes, MRIs, PET scans, blood and urine collections worthwhile.
After all, how many people can say they are truly part of making scientific history? It’s a good feeling.
Phil Gutis is a former New York Times reporter and current Being Patient contributor who was diagnosed with early onset Alzheimer’s. This article is part of his Phil’s Journal series, chronicling his experience living with Alzheimer’s.