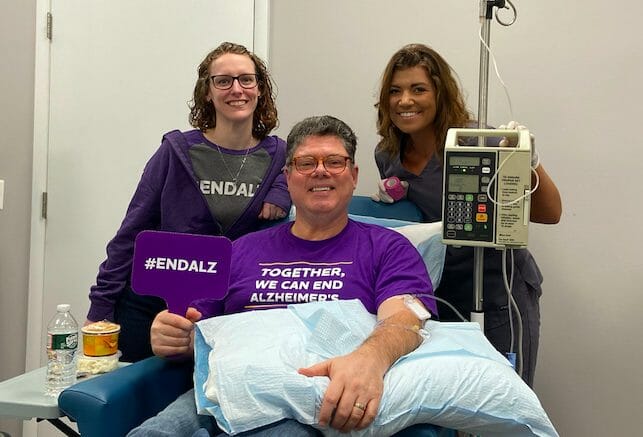
Geri Taylor, a participant in the aducanumab (Aduhelm) clinical trial, and her husband Jim share insights about taking part in the research.
UPDATE: 3 March 2024, 8:38 P.M. ET. In February 2024, Biogen took Aduhelm off the market, citing financial concerns. Although the drug did receive accelerated, conditional FDA approval for the treatment of early Alzheimer’s disease in 2021, it is no longer available to new patients. The company announced it would sunset trials in May 2024 and cease supplying the drug to current patients in November 2024.
During her time as a clinical trial participant, receiving monthly infusions of aducanumab — approved by the FDA in June 2021 as the first disease-modifying treatment for Alzheimer’s, brand name Aduhelm — became routine for Geri Taylor, a retired nurse and health administrator living with Alzheimer’s.
Aduhelm, a human antibody-based drug, targets the hallmark of Alzheimer’s: beta-amyloid plaques that build up in the brains of people with the disease. The last Alzheimer’s treatment the FDA approved was nearly two decades ago, and before aducanumab, no disease-modifying drug had ever made it through the final stages of clinical trials. Its approval, however, has been controversial.
Aducanumab Primer: What Is It
and How Does It Fight Alzheimer’s?
Looking for answers about the investigational
Alzheimer’s drug aducanumab? Here’s our explainer
on what it is, what it does, and more, according to experts.
Geri first enrolled in the clinical trial of aducanumab in 2015, making two-hour long drives with her husband Jim from their home to Yale-New Haven Hospital.
“I felt like I was driving home with Madame Curie in the car,” Jim told Being Patient in reference to the Nobel laureate Marie Curie, “because she [Geri] was there on the bench with the researchers doing all she could to find a breakthrough treatment.”
Aducanumab, however, has gone through a convoluted path leading up to the FDA’s much-anticipated decision. Geri and fellow participants were taken off of treatment when Biogen and its partner Eisai abruptly halted aducanumab’s two Phase 3 trials in March 2019 following a “futility analysis.”
Then, in another twist, FDA advisers recently rejected evidence supporting the benefits of aducanumab. From here, the drug’s future is uncertain. With less than a week before the deadline for FDA’s decision, Being Patient reporter Nicholas Chan spoke with Geri and Jim about their journey with Alzheimer’s and the clinical trial of aducanumab.
“I thought that I had the ability to not only
help myself, but also my family and generally
people living with Alzheimer’s.”
Nicholas Chan: Geri, you were first diagnosed with mild cognitive impairment in 2012, which is the precursor of Alzheimer’s. How did you first know something was wrong?
Geri Taylor: I got off the subway and didn’t know why I was there. I was going someplace else, of course. I couldn’t remember that. There were a couple of other hallmarks, the most dramatic of all was when I was going to fix my hair, and I didn’t recognize my own face.
I knew through my family that I was most likely to get Alzheimer’s. I was a nurse and I also knew what it was going to look like. That being said, it was still a hit, and the hit for me was figuring out how to live with it, and most importantly talking to Jim [about] how that was going to go. Because of my background in the healthcare arena, the living part of it was easier for me in the beginning.
NC: What motivated you to join a clinical trial?
GT: I thought that I had the ability to not only help myself, but also my family and generally people living with Alzheimer’s.
Jim Taylor: We knew that to be in an interventional trial, you’re going to get supplemental care and, often, better care than visiting the neurologist once a year, and also that we would have a chance for the possible drug that might really make a difference. But something most people don’t think about is there’s a tremendous psychological advantage to being in a trial, as you know you’re helping the research, and you’re doing your best to manage the disease as opposed to suffering from it.
Aducanumab Approved:
U.S. FDA Gives Biogen
Alzheimer’s Drug Approval
NC: How often would you have to go to the clinical trial site?
JT: We would go once a month, and Geri would spend an hour with the needle in her arm, infusing active medication into her body. You had to wait an hour afterward before they release you. So it was a two-and-a-half to three-hour time in the institution. In addition to that, we had to go for quarterly MRIs and PET scans.
NC: Geri, you are now back to receiving infusions of aducanumab since clinical trials of the drug have been relaunched. Can you tell us what it’s like being a participant? Is it demanding?
GT: Not at all. The person who has the load is Jim, managing to get us there on time, that kind of thing. During the [infusion], you’re just resting or reading, talking to the staff that comes by and chit-chatting. I got friendly with a few of them.
JT: During COVID, it was our one social opportunity. It’s the staff you become attached to, and they very much want to be social and want you to feel connected, because that keeps people in [clinical trials]. One of the difficulties with clinical trials is keeping people in longer-term trials. People drop out, so retention is a concern. The staff wants to build as much personal connection as possible. That’s very reassuring, because they know what they’re doing.
“Something most people don’t think about
is there’s a tremendous psychological advantage
to being in a trial, as you know you’re helping the
research, and you’re doing your best to
manage the disease as opposed to suffering from it.”
NC: From your personal experience, do you think aducanumab may have been beneficial?
JT: Before the trial was interrupted, Geri was on the trial for about three years. She declined very slowly. We really thought the drug was helping. Of course, there’s no way to know that, but her ability to maintain normal daily activities and live and take care of herself was really excellent. We traveled all the time and she managed all that well.
During what I call the interregnum, the period when she wasn’t getting the medication, she started to decline more quickly. Now that she’s back on the drug, I kind of doubt it’s helpful. The benefit is really to get started as early as possible … She’s in the stage in which decline is noticeable, and it’s much more rapid. I don’t like to speak for Geri and would never have [had to] two or three years ago, but now I try to give her words when she’s struggling a little bit.
“What I always want to say to people is, ‘Wait a minute,
there is some really positive news coming out of …
trials of other drugs … We’ve never been in that kind of situation
before, and that’s the hope I want people to focus on.”
NC: Are there any aspects of being in the clinical trial that aren’t pleasant?
JT: The thing that maybe is not pleasant is Geri has to take neurological testing quarterly. It’s not particularly pleasant to have it reinforced that you’re losing your memory, that you’re dying. They often give you two or three tests, and they do it every quarter.
GT: [It’s like] when you go to school and they give your report card and [your grades are] always going down.
JT: When they read a list of 20 words and they ask you to then repeat back as many words as you could remember, Geri sees that every quarter, she’s getting fewer and fewer [words]. That’s really tough.
NC: Both of you spoke during the public comment section of the November 2020 meeting where FDA advisors rejected evidence that supported aducanumab. What do you make of their decision?
JT: I think the scientists were honest and I think they backed their opinion, but I don’t think that should be the final word, which it clearly isn’t. Billy Dunn, [director of neurology products for the FDA’s Center for Drug Evaluation and Research], has to figure that out.
But Biogen made a very bad mistake, and I think they have paid the consequences and are paying the consequences … They really messed up the data and they’ve put the FDA in a very, very difficult spot. I don’t hear Biogen acknowledging that very often.
I do think a lot of the criticism [stems from the fact that] the scientists are reading the incomplete data and saying, ‘How do you make a decision on this incomplete data?’ That’s what the FDA will have to decide and that’s what Biogen kind of handed them.
Want to learn more about clinical trials
for Alzheimer’s and dementia?
Check out the Lilly Trial Guide.
NC: Soon enough, we’ll know whether the FDA approves aducanumab or not.
JT: It may not be approved. A lot of people will be disappointed, but what I always want to say to people is, ‘Wait a minute, there is some really positive news coming out of … trials of other drugs. Donanemab is coming. BAN-2401 is very exciting. Green Valley has oligomannate. [With aducanumab], that’s four potential disease-modifying treatments. We’ve never been in that kind of situation before, and that’s the hope I want people to focus on.
The interview has been edited for length and clarity. This article was updated Dec. 21, 2021 to reflect aducanumab’s FDA approval.
Contact Nicholas Chan at nicholas@beingpatient.com




I am interested in any trials around the Kansas City area. My (80 yr) husband had a double hemorrhagic stroke 2 yrs ago and has alzheimers. I am 77 but notice a cognitive decline. Ginger Baird.
Heartfelt and great testimonial. But, science is not base on isolated cases or testimonials. We have to see what the RCTs will show before we can rely on any real data.
I would be very interested in enrolling in the Aducanumab Study. I have been diagnosed with MCI and I want my illness not to progress.
my Father has memory issues, I’m interested in any new clinical trials and drugs to hold on to his memory as long as he can.