The response was disappointing but not particularly surprising.
Earlier in the day, I had sent a quick email to check in with the staff at Advanced Memory Institute of New Jersey to see if they’d made any progress on transferring my participation in Biogen’s new trial for aducanumab to their site.
The response came quickly. “The good news is that it will definitely not be a problem,” wrote Anna Brocco, Director of Operations for Advanced Memory.
“The bad news is that in light of the current pandemic, we are now closed for at least the next two weeks,” Brocco said. “We will have you added to the schedule no problem when we return.”
My experience is apparently being repeated around the world as COVID-19 crushes all types of activity. STAT News reports that COVID-19’s disruption of clinical trials is becoming an almost daily ritual.
“The scope of the clinical trial delays and suspension is hard to quantify,” STAT reports. “But consider this snapshot: biotech and pharma companies are currently running more than 120 Phase 3 clinical trials with top-line data read outs expected before the end of the year.”
Biogen is one of those companies. It is preparing to seek FDA approval for its experimental drug aducanumab, which has shown promise in slowing down the impact of Alzheimer’s, particularly for those of us who are in the earliest stages of the disease.
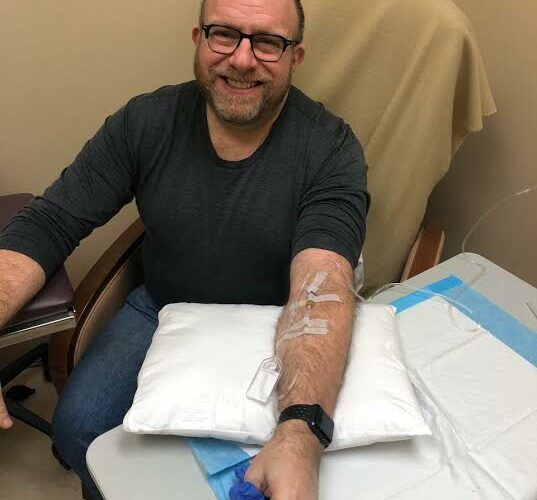
While it awaits a decision from the FDA, Biogen has launched a new trial called EMBARK for people who were enrolled in earlier phase 3 trials of aducanumab. The first-in-the-world infusion for the EMBARK study took place about two weeks ago at Advanced Memory.
I interviewed Being Patient advisor Jeff Borghoff as he received his infusion and was so impressed with the team at Advanced Med that I asked to have my trial participation shifted to their Tom’s River location.
It wasn’t that I had any concerns about my previous trial center. In fact, I loved being part of the aducanumab trial at the University of Pennsylvania’s Memory Center.
But earlier in the month I had received a note from the Penn Memory staff that they were unlikely to be able to launch the EMBARK study until late May or early June at the earliest. And that was before the pandemic struck.
Advanced Memory told me that they thought they could get my infusions started by the end of March or early April. So I made the decision to enroll in EMBARK at Advanced Med and filled out all the necessary paperwork.
Then COVID-19 began to wreak havoc. I spoke with Brocco from Advanced Memory and could hear in her voice how hard it was to make the decision to close shop.
“It was hard, we’ve never had to do this before,” Brocco said. “We decided to take two weeks and then re-evaluate where we’re at then. We don’t want to be part of the chain of transmission.”
The considerations the clinic staff took into account included the age and the underlying health conditions of most of its patients, the impossibility of obtaining protective masks and other gear and the very essential fact that an infusion cannot be done while remaining six feet from a patient.
The decision to close, Brocco said, ultimately came down to a very practical consideration—the clinic was unable to obtain even the most basic cleaning supplies.
“There’s been such a run on everything,” she said. “We were running out of the basic supplies to keep the office clean and open. I can’t even get this stuff for my house. How could I expect to get it for the office?”
A spokesman for Biogen said that the company is continuing its worldwide clinical trials but “expects that COVID-19 precautions may impact the timeline of some of our trials.”
“We also understand and defer to healthcare professionals on patient- and site-level decisions about visits during this difficult time, as practices are adapting to local guidelines regarding social distancing, quarantines and slowing the spread of COVID-19,” spokesman David Caouette said.
To get another perspective, I also spoke with Dr. Sharon Cohen, Medical Director at the Toronto Memory Program, which is Canada’s most active clinical trial center for Alzheimer’s and other dementias.
While their memory clinic has gone virtual, Cohen said the research portion of their program continues for the time being.
In addition to doing more rigorous cleaning and sanitizing, Cohen said the clinic has been aggressively screening patients to make sure they haven’t travelled out of the country recently and are feeling well before allowing them to come in for appointments.
The clinic is leaving doors open when possible so people don’t have to touch knobs, Cohen said, and added that the Uber cars are being used to pick up staff who normally commuted to the office by public transport.
The local government has decided that clinical research services are among essential businesses that are allowed to continue to operate.
“We have let all of our sponsors know that we are open and most of the sponsors are very happy to continue with their research visits and the start-up activities,” Cohen said.
“I’m saying that today,” she said, “but of course everything could change by tomorrow.”
Phil Gutis is a former New York Times reporter and current Being Patient contributor who was diagnosed with early onset Alzheimer’s. This article is part of his Phil’s Journal series, chronicling his experience living with Alzheimer’s.