In a surprising turn of events, the Advisory Committee of experts on Biogen’s experimental Alzheimer’s drug aducanumab recommended against FDA approval of the drug on Friday. Being Patient reporter Phil Gutis, who participated in the aducanumab Phase 3, trial reflects on the controversy and concludes that another trial is necessary.
I trust science. Always have. I believe climate change is real. I wear a mask whenever I’m in a store or walking outside in a crowd. I really don’t want to have to deal with a bout of COVID, and I will eagerly line up for a vaccine once the scientists tell us it is safe and effective.
My continued willingness to follow the science, however, was sorely tested on Friday when I spent six-plus hours listening to professors of neurology, biostatistics and medicine trashing the results of large clinical studies of aducanumab, an Alzheimer’s drug being developed by Biogen and Eisai.
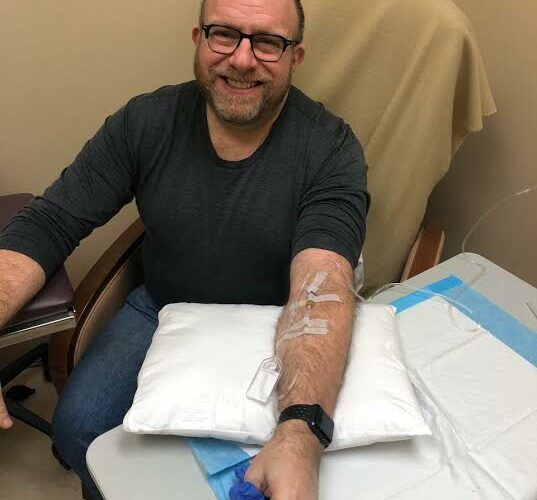
I was a participant in one of those clinical studies and continue to receive aducanumab as part of another ongoing trial.
Each month, I travel about 90 minutes each way to the trial center. Once there, I hold my breath while a nurse pokes at my arm and hooks me up to an IV. On a whole, the experience has been very positive — I’ve met tons of caring wonderful people — but there have been moments when I questioned my sanity.
I did not tell my story on Friday but listened carefully as people living with Alzheimer’s and their caregivers lined up during the public comment section of the hearing to offer their sincere belief that aducanumab worked in slowing their cognitive decline.
I also listened carefully as the Alzheimer’s Association — I serve on the Board of the association’s Delaware Valley chapter — also made the case for why aducanumab should be approved.
“While everyone experiences the disease differently, the trajectory of cognitive and functional decline is currently inevitable,” said Joanne Pike, the Association’s Chief Strategy Officer.
“For individuals living with Alzheimer’s, they lose more of themselves as it progresses. It’s not just memories they lose. They lose the ability to participate in the world around them. They lose their independence.”
Once again, I did not speak at the hearing. But I can definitely say that it is my belief that aducanumab has indeed slowed the progression of my Alzheimer’s.
After experiencing memory concerns — my family says that I complained that “something just wasn’t right” — I was diagnosed with early onset Alzheimer’s through the Biogen trial. Five years after that diagnosis, I continue to suffer from a moderate case of mild cognitive impairment. But I definitely feel and perform better than I did after diagnosis.
I definitely have my struggles. But I am still able to do some fairly high-level work. After all, I covered a six-hour-plus tele-hearing of the FDA experts and delivered on deadline a thousand-word piece for Being Patient about their deliberations.
But unlike in my prime — when that kind of assignment was not a big deal — drafting that article was pretty much all I did for days afterward. I was beyond exhausted.
Sitting with my husband last night — a full 48 hours after the hearing concluded — I became deeply frustrated with my inability to come up with the words to describe the half-hour of clean- up work I did in the yard.
Fighting tears, I laid in bed after dinner, completely incapable of doing anything more than playing solitaire on my iPad.
Biogen and Elsai Alzheimer’s Drug Trials 301 and 302
I recognize that my stories and the testimony offered during the hearing are at best anecdotal. They are just as scientifically irrelevant as President Trump’s discredited trumpeting of the malaria drug hydroxychloroquine as a treatment for COVID. Or his supposition that infusing COVID patients with bleach could be a cure. (It’s not!)
In contrast, the debate during the FDA hearing was focused like a laser on statistical probability. Frankly, I found the conversation challenging to follow. So I’m going to rely on an excellent report from the website of Science Magazine to explain.
After preliminary trials of aducanumab were well- received, Biogen and Eisai launched two identical phase three clinical trials of the experimental drug in patients with early stage disease.
These two trials, called studies 301 and 302, have “caused confusion and controversy,” Science wrote.
In fact, in March 2019, based on what’s called a “futility analysis,” the pharmaceutical companies cancelled both trials in March 2019.
Six months later the companies shocked the Alzheimer’s community by announcing that late-arriving data for study 302 had in fact shown positive results in reducing cognitive decline for people on the drug and said that based on that analysis, they would seek FDA approval of aducanumab.
Study 301, however, was still negative. In fact, patients in that study showed a worse decline than people who were receiving a placebo.
Biogen and the FDA staff are pushing for Aducanumab’s approval based on study 302 alone. And that did not sit well with the independent reviewers.
Advisory Committee Recommends Against FDA Approval for Aducanumab
Scott Emerson, a biostatistician from the University of Washington, told the hearing that the two trials were meant to be evaluated together. “We may never, ever, ever ignore the fact that study 301 was done,” he said.
At the end of the day, asked by the FDA whether it was “reasonable to consider Study 302 as primary evidence of effectiveness of aducanumab for the treatment of Alzheimer’s disease,” the committee overwhelmingly said no. Only one member, committee chair Nathan Fountain, a University of Virginia neurologist, voted uncertain. Not one committee member voted yes.
Jason Karlawish, the co-director of the Memory Center at the University of Pennsylvania (and one of my neurologists), said that he is unaware of a time when the FDA has “ever issued a decision on an application that was the opposite of the recommendations of its advisory committee.”
“With these facts,” Karlawish added, “FDA seems destined to reject the application. If it does, then Biogen will need to decide whether to conduct another clinical trial.”
In many ways, what Dr. Karlawish outlines has been the scientific consensus ever since Biogen revealed its initial data to a scientific conference in San Diego last year.
Trusting Science
And after a lot of thought this weekend, I think it is probably the right call. We have to trust the science, and because of the unfortunate decision to end the trials early, Biogen and Eisai should in fact be required to do another large clinical trial.
My only hope — and I recognize this sounds enormously selfish — is that those of us who have participated in the original aducanumab trials will be allowed to continue in the ongoing open- label trial.
After all, the 4,000 people who offered up their arms once a month for the last five years helped develop this drug. And we’ve already lost the treatment once following the futility analysis.
Want to learn more about clinical trials
for Alzheimer’s and dementia?
Check out the Lilly Trial Guide.
To once again take away a treatment that — at least anecdotally — seems to be doing so much good would just be cruel.
So let science proceed. The FDA should require Biogen to undertake a new Phase 3 trial and through that offer the experimental treatment to thousands more people. The cost will be significant, yes, but Biogen stands to make billions and billions of dollars if the company’s analysis is ultimately proven correct.
But please please please don’t forget the folks who’ve brought us this far. Keep our open- label trial going, and let us continue to benefit from the drug.
Phil Gutis is a former New York Times reporter and current Being Patient contributor who was diagnosed with early onset Alzheimer’s. This article is part of his Phil’s Journal series, chronicling his experience living with Alzheimer’s.



Abducanumab should definitely be given another trial due to the relevant debate of it’s potential efficacy !