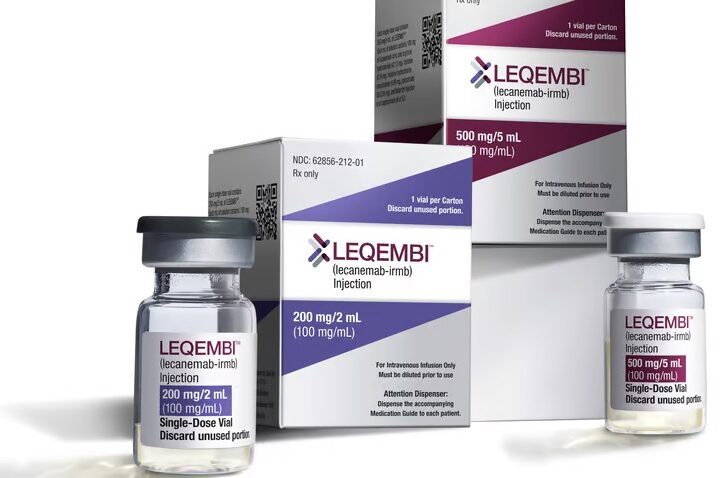
A second patient has died of brain bleeds — an anti-amyloid side effect — during the clinical trial extension of Eisai's Alzheimer's treatment lecanemab (brand name Leqembi).
Update 27 January, 2023: In January 2023, lecanemab was granted accelerated approval by the FDA and made available to patients under the brand name Leqembi. Per the FDA’s accelerated approval program, trials to confirm is benefits to patients are ongoing.
A 65-year-old woman with early Alzheimer’s suffered a fatal a brain bleed while receiving Eisai’s experimental Alzheimer’s antibody treatment lecanemab.
At the end of September, Eisai and Biogen announced that their experimental anti-amyloid drug lecanemab successfully slowed cognition in Alzheimer’s patients, according to data from Clarity AD, the drug’s Phase 3 trials. In late October, it was reported that an 80-year-old participant in the lecanemab trial died during the trials in June, also of a brain bleed.
According to an unpublished case report obtained by Science magazine, in the case of this second participant death, the woman had received infusions of lecanemab during the trial. She suffered a stroke and experienced brain swelling and bleeding known as amyloid-related imaging abnormalities (ARIA).
ARIA are a known side effect of anti-amyloid Alzheimer’s drugs and have been seen in patients taking the drugmaker’s previous ant–amyloid Alzheimer’s treatment, Aduhelm. In Aduhelm, ARIA was often mild and asymptomatic, and occurred in about 40 percent of participants.
These two deaths during trial extensions are fueling further safety concerns about Alzheimer’s anti-amyloids and their risks, particularly when combined with other conditions or interventions.
(In February 2024, Biogen took Aduhelm off the market indefinitely.)
How many people have died during lecanemab Phase 3 clinical trials?
In total, Eisai has reported that 13 participants died over the course of the trial, divided between the two groups with no statistically significant difference between test and placebo. No details around the causes of those deaths have been made publicly available, so none of these deaths, even those among people taking lecanemab, can be definitively linked to the drug.
According to a Biogen investor update, in the core Phase 3 study and in the open-label extension study that followed, there was no statistically significant difference between the group that was given lecanemab and the control group, which was given a placebo, in terms of the incidence of cerebral macrohemorrhage. In both the test and control groups, rates of death involving cerebral macrohemorrhage were 0.1% — one of 897 patients in the placebo group, and two of 1,608 patients in the lecanemab group.
Both of these deaths while on lecanemab have occurred during this open-label extension study, according to Biogen. “Both cases had significant comorbidities and risk factors including anticoagulation contributing to macrohemorrhage or death,” the update read. “Therefore, it is Eisai’s assessment that the deaths cannot be attributed to lecanemab.”
Despite Eisai’s assessment, these two deaths during the trial extension, among patients who are taking lecanemab, both involving brain bleeds, are drawing media attention because they may speak more directly to the risks of anti-amyloid drugs, particularly when combined with existing conditions or medical interventions, such as blood thinners.
The risk of combination of blood thinners and anti-amyloids
In response to the woman’s stroke, emergency room personnel administered a tissue plasminogen activator (tPA) to address blood clots — a common stroke intervention. This step seemed to spur bleeding in her brain’s outer layer, according to the case report as described by Science.
In Science’s interviews with her husband following her death, and in notes from her case report, the event was extremely dramatic.
“As soon as they put it in her, it was like her body was on fire,” her husband told Science of the administration of the tPA. “She was screaming, and it took like eight people to hold her down. It was horrific. Everybody’s running in and [asking] ‘What the hell is going on?’”
She experienced seizures, was put on a ventilator, and died a few days later.
The Eisai ClarityAD trial consent form, which is 30 pages in length, does contain a warning about blood thinners and their association with a heightened risk of brain bleeds, according to Science.
Deaths in Alzheimer’s trial drive drug safety questions
It was unclear after the first death whether or not the hemorrhaging was caused by the drug trial, and the link remains unproven. Regardless, this second death among trial participants, in line with both the first death and the risks of anti-amyloids, intensifies questions around lecanemab’s safety and around how widely it should be prescribed if it does indeed secure FDA approval.
This summer, it was granted “breakthrough status” by the FDA, ensuring it priority review by regulators. At that time, the FDA had expressed an intention to announce their decision by January 6, 2023.
Neuropathologist Rudolph Castellani at Chicago’s Northwestern University Medical Center, who studies Alzheimer’s, conducted an autopsy at the request of the husband of the woman who died during the trial.
Castellani shared his personal opinion on the case with Science: “There’s zero doubt in my mind that this is a treatment-caused illness and death,” he said. “If the patient hadn’t been on lecanemab, she would be alive today.”
Individual opinions aside, the brain is a complicated thing and Alzheimer’s remains little understood. Researchers still don’t know for certain whether beta-amyloid is even the cause of the disease.
Pegging the combination of a blood thinner and an anti-amyloid as a cause of death is not possible at this point, according to Holman, “The available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause,” Holman said.
ClarityAD trials will continue to gather data on the drug’s efficacy at reducing cognitive decline in people in early stages of Alzheimer’s disease.
Additional reporting by Simon Spichak
UPDATED Nov. 29, 05:13 A.M. E.T.: This article was updated to include exclusive interview with an Eisai spokesperson on the issue.
CORRECTION Dec. 23, 09:09 A.M. E.T.: This article was updated to specify that the death reported here was part of trial extensions, and that during the initial Phase 3 trials for lecanemab, 13 deaths were divided fairly evenly between the placebo and test groups. Eisai has stated there was no statistically significant difference between deaths in the group taking the drug vs. the group taking the placebo. No specific information has been made publicly available as to the cause of the 13 trial deaths.
UPDATE: 3 March 2024, 9:20 P.M. ET. In February 2024, Biogen took Aduhelm off the market, citing financial concerns. Although the drug did receive accelerated, conditional FDA approval for the treatment of early Alzheimer’s disease in 2021, it is no longer available to new patients. The company announced it would sunset trials in May 2024 and cease supplying the drug to current patients in November 2024.