Alzheimer's drug Leqembi is fully approved in the U.S. and several Asian countries. In the UK, EU and Australia, access is a different story.
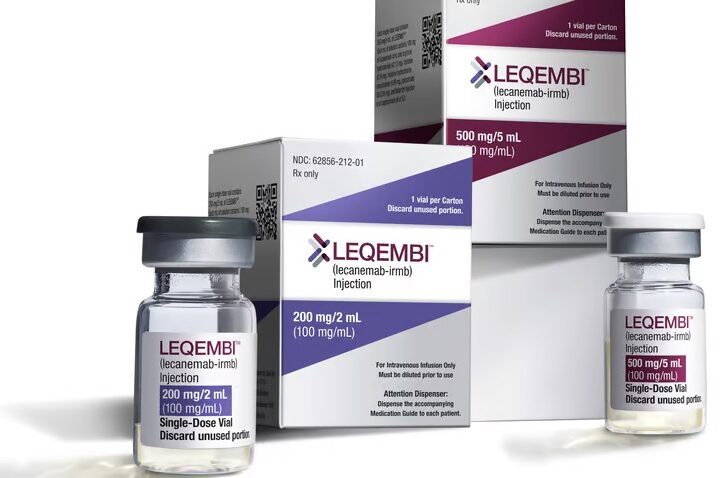
Eisai and Biogen’s Alzheimer’s drug Leqembi (generic name lecanemab) was the first fully-FDA approved disease-modifying treatment for early-stage Alzheimer’s disease in United States history, as of January 2023. This year, Kisunla followed. Both are monoclonal antibodies designed to break down amyloid plaques in the brains of people with early-stage Alzheimer’s disease.
Shortly after Leqembi hit the market in the U.S., Japan, South Korea, China, Hong Kong, Israel, U.A.E., Mexico, and Macau also greenlit the drug. But, throughout its trials and into its rollout, it has remained controversial, as clinicians offer up conflicting opinions about its safety and efficacy, and question whether this new class of drug is really making a clinically meaningful difference for patients in slowing cognitive decline. In 2024, regulating agencies in other countries and regions have flat-out declined to bring the drug to market. Some changed their opinion upon appeal.
However, Australia’s regulatory body will not be overturning its recent October 2024 decision to reject Leqembi. The agency offered Eisai and Biogen a concession that would prevent most people with early Alzheimer’s from accessing the treatment. The drugmakers said no, and Leqembi remains off the market for people in Australia, at least for now.
Is Leqembi available in Europe?
This decision is pending as of November, 2024, but it’s looking like yes: In July 2024, Europe’s regulating body, the European Medicines Agency, declined to approve Leqembi, meaning that outside of clinical trials, it won’t be available to patients in Europe. But then, in November, they reversed that decision.
“The benefits of treatment are not large enough to outweigh the risks associated with Leqembi,” the regulators wrote in a statement on their July decision. And in November, they decided, instead, that in people without a copy of ApoE4, “the benefits of Leqembi in slowing down progression of symptoms of the disease are greater than its risks.”
Is Leqembi available in Australia?
In October 2024, Australia has also rejected the Alzheimer’s drug. Regulators wrote that they made their decision on the basis that “the demonstrated efficacy did not outweigh the safety risks.”
Australia’s regulating body — the TGA — declined to approve the drug, and in their announcement, called out clinical study data which shows the improvement in cognitive decline rates for people on Leqembi isn’t considered clinically meaningful. In other words, it makes very little measurable difference in cognitive health. They also expressed concern about the much-discussed side effect of ARIA — small brain bleeds, for which people who carry genetic biomarkers for Alzheimer’s and people on certain medications are especially at risk.
This decision was supported by independent expert advice from the TGA’s Advisory Committee on Medicines.
In February 2025, they reviewed their decision. Australia’s regulatory body conceded that the drug is safe and effective for patients without any copies of the ApoE4 risk gene, which increases the chances of ARIA. The committee offered to approve the drug for this slice of the population, but Eisai declined the offer.
“This indication would deny approximately two-thirds of all potentially eligible patients access to a treatment that could slow the progression of Alzheimer’s disease,” Lynn Kramer, MD, chief clinical officer at Eisai said in a press release, adding that the company is exploring its options to appeal the decision.
Is Leqembi available in the UK?
In August 2024, UK’s drug regulation agency — UK Medicines and Healthcare products Regulatory Agency — approved Leqembi for early stage Alzheimer’s who have zero or one copy of the Alzheimer’s risk gene ApoE4. But, UK patients won’t have an easy time getting access to it, because UK Health Services, country’s state-run insurer, said the drug “cannot be considered good value for the taxpayer” due to its small therapeutic benefits and big risk for side effects.
The UK has socialized healthcare and covers drugs for patients. But according to draft guidance by the country’s healthcare regulator, National Institute for Health and Care Excellence, NICE isn’t planning on covering Leqembi because the drug’s treatment benefits to patients are considered too small, and on top of that, NICE says, the long-term impact of the drug is unknown. The drug would cost tens of thousands of pounds per year out of pocket.
“The NICE decision is likely to be disappointing to those affected by Alzheimer’s disease whose hopes may have been elevated by the early promise of amyloid-targeting therapies,” Paul Morgan, the interim director of the UK Dementia Research Institute at Cardiff University said in a comment on the Science Media Center. “However, given the many unanswered questions around patient selection, monitoring, long-term impact and side effects, the ‘wait and see’ approach is understandable.”
The draft guidance also recommends against administering the drug to people who have two copies of the ApoE4 gene.
NICE’s ruling was not final at the time of issuance. The drug review committee is currently reviewing public comments on the draft guidance and will reconvene to decide whether to stand its ground.
As for future anti-amyloids, rumors were circulating as of August 2024 that MHRA does not plan to approve donanemab (recently greenlit by the U.S. FDA and put on the market as Kisunla).
UPDATED 6 May, 2025: This article was updated with new information about Australia’s regulating agency’s ruling on Leqembi.



I am of the opinion that people suffering Alt. should at least be given the opportunity of trying the drug after it is made clear to them the possible side affects, the possible safety de affects and cost, thus allowing them to at least make a choice. If cost was an issue I think people would be prepared to self fund.
Hi there, thank you for reaching out and sharing your opinion. We’re wishing you the best as you continue to navigate this journey.
How is Kinsula and Leqembi different from the approve Donepezil HCL
Hi Tina, thank you for reaching out. Leqembi and Kisunla are designed to target a toxic form of beta-amyloid, a protein that’s thought to be involved in disease progression. These drugs help clear out toxic forms of beta-amyloid and are shown to slow the course of the disease by a small amount. They’re prescribed for people with mild cognitive impairment who have beta amyloid in their brain and early Alzheimer’s. Donepezil, on the other hand, is a type of drug designed to treat symptoms like memory loss but doesn’t slow the course of the disease. It’s for mild to moderate Alzheimer’s, comes in pill form and it’s fairly cheap. The cost of all these drugs is covered in part by Medicare. For people under 65, their private insurer may not cover the cost of Leqembi or Kisunla. Take care!