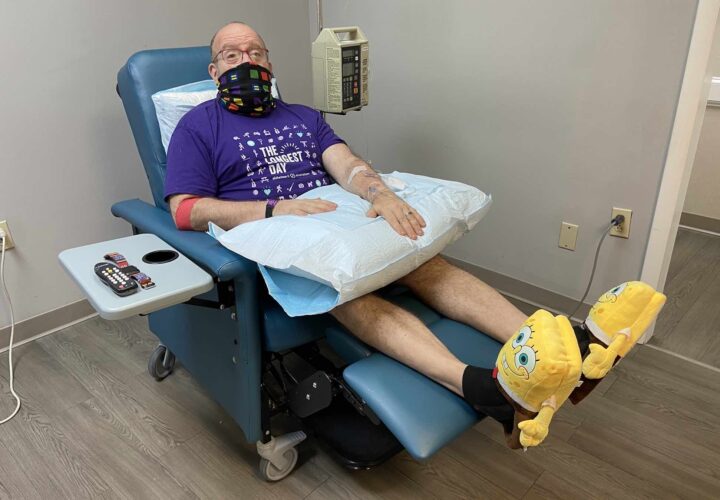
Image: Phil Gutis receives an aducanumab infusion while carrying out his “silly shoe challenge.”
Phil Gutis reflects on the significance of participating in the research of the closely-watched experimental drug for Alzheimer’s — regardless of the FDA’s decision on whether or not to approve it.
There are approximately 3,500 very special people around the world who are eagerly awaiting the FDA’s decision, expected on or before June 7, on the fate of aducanumab, the first drug to show promise in slowing the progression of Alzheimer’s in people in the earliest stages of the disease.
These people are not the thousands of researchers and doctors working to find treatments for neurodegenerative diseases, although I’m sure they are watching very, very closely. Nor are they the hundreds of thousands of shareholders and employers of Biogen and Eisai, the companies that have pumped untold millions into the development of aducanumab.
No, these 3,500 special people are the volunteers who have offered their arms up each month to have a mystery bag of fluid slowly dripped into their veins. They have endured quarterly MRIs and annual PET scans. They’ve been repeatedly quizzed on their cognitive skills and memory.
I’m one of those folks. For most of the last five years, I’ve been a subject — number 658-021 — in a grand research study that has been twistier than the most elaborate roller coaster.
I watched sadly and helplessly in the spring of 2019 when Biogen and Eisai cancelled aducanumab trials because, they said, the drug failed a “futility analysis.” Then I listened with hope — and more than a bit of anger and disbelief — nine months later as the companies essentially said “whoops.” Late-arriving data, they explained, suggested that aducanumab was slowing the progression of Alzheimer’s by 22 percent in people (like me) in the earliest stages of the disease.
A few months after that announcement, I flew to San Diego as a reporter for Being Patient and sat in a crowded hotel ballroom desperately trying to follow along as Biogen’s scientists reported on reams of data that, in essence, boiled down to a very simple message: Trial participants who received the highest dose, for the longest time, had the best outcomes. (I was receiving a medium dose before the trial was cancelled.)
I actually ran into a friendly face at the San Diego conference. Dr. David Wolk, the co-director of the Memory Center at the University of Pennsylvania (and my neurologist), was one of the 1,600 researchers in the crowd. I stumbled on him while he was seeking caffeine during a late-afternoon break in the proceedings.
His expert opinion was that aducanumab works. “How well it works is debatable,” he told me. “And in a world in which there tends to be a degree of conservatism about approving very expensive drugs that also have side effects, I think it is unlikely that it will be approved without additional data being required.”
I wasn’t surprised by his caution. Many of the researchers in the audience had expressed at least some skepticism as the day proceeded. There was deep wariness about the data mix-up and many researchers seemed to believe that another trial was necessary before the government approved the drug for general use.
Months later, the FDA convened a highly anticipated advisory panel of scientists and statisticians to weigh in on aducanumab — their recommendation would have serious bearing on the FDA’s forthcoming decision. The panel meeting was held during the height of the pandemic, so rather than squeezing into another ballroom, thousands of folks virtually jammed into a Zoom call where we all discovered that, as Dr. Wolk foreshadowed, these scientists were not persuaded as to aducanumab’s efficacy.
At the end of a very long day, the panel voted almost unanimously — there was just one researcher who abstained during the final vote — to recommend that the FDA not approve aducanumab, because they were not persuaded it would be effective.
Instead, they suggested, the companies should launch another Phase 3 trial of the drug.
In an essay following the advisory committee‘s recommendation, I took a controversial position among the Alzheimer’s advocate community: the FDA should listen to its panel members, “follow the science” and require another study. I also noted that I selfishly hoped that the 3,500 people who had already been in the trials would be allowed to continue to receive the drug.
Want to learn more about clinical trials
for Alzheimer’s and dementia?
Check out the Lilly Trial Guide.
Since then, I’ve come to learn that yet even further analysis of the trial data has now buttressed the case for some sort of approval. Many observers expect the FDA to give aducanumab some kind of conditional approval. Perhaps the government will give the companies the okay to begin marketing the drug, while at the same time requiring the companies to complete what is called a Phase 4 trial.
Phase 4 trials, also known as post-marketing surveillance, are conducted after a drug is already available to the general public. The main goal of a Phase 4 trial is to make sure the drug is working in the real world, to keep studying any long-term risks and benefits and to suss out any rare side effects.
Anecdotally, I believe that aducanumab has helped me. I feel much better than I did five years ago. Truthfully, five years ago, I didn’t expect to still be alive today.
On the negative side, the memories that I’ve lost have not returned, and I continue to stumble across activities that seemingly were never written into my memory banks. Just this week, my husband, Tim, asked if I remembered hiking around a quarry where I used to work.
I looked at him blankly. He explained further. Still nothing. I’ve since thought and thought and thought. Nothing. Unfortunately this is a pretty common occurrence. Restaurants, theater, comedy shows, previous work travails. Memories of pets that have left us. So much is gone.
But I’ve also learned through a longitudinal study PET scan that I no longer have any amyloid in my brain. A protein normally found in the brain, amyloid is believed to somehow grow uncontrollably during the Alzheimer’s disease process leading to brain cell death and atrophy.
The scan, taken about two years ago as part of the Aging Brain Cohort study at the Penn Memory Center, confirmed my growing inklings that aducanumab was indeed helping me. I began to feel like I was emerging from a constant mental fog.
In addition to beginning to write for Being Patient, I boosted my work as a volunteer with the Alzheimer’s Association and am now proud to serve on the board of the association’s Delaware Valley Chapter and to co-chair the chapter’s Longest Day campaign.
I’ve also slowly creeped back into the world of work. I’m working a few hours a week editing court transcripts for a small group of court reporters. While I’m certainly not going to get rich doing this work, I can’t express how important it is to feel useful again. To feel like I have a job to do and to have pride in work well done.
The scan confirmed my growing inklings that aducanumab
was indeed helping me. I began to feel like I was
emerging from a constant mental fog.
Given the twisty history of aducanumab, I’m wary of getting too hopeful that the FDA will approve the drug. And the questions that will emerge if the FDA does approve the drug are overwhelming in themselves: How much will the drug cost? (Some estimates guess that could be as much as $50,000 per person annually.) Will Medicare and Medicaid and private insurers cover aducanumab treatment? Will aducanumab eventually become a pill that you pop with breakfast, or will patients need to travel to infusion centers for intravenous drips?
Overall, no matter the outcome, I’m deeply proud to have been part of what may very well become medical history: the first-ever drug to treat the underlying conditions that lead to Alzheimer’s.
Even if the FDA somehow rejects aducanumab, I hope the world doesn’t forget the 3,500 volunteers in the aducanumab trials — and the tens of thousands of other volunteers in other previously unsuccessful trials — who have gotten us to the precipice of history.
Phil Gutis is a former New York Times reporter and current Being Patient contributor who was diagnosed with early onset Alzheimer’s. This article is part of his Phil’s Journal series, chronicling his experience living with Alzheimer’s.


Im a 68 yr old female. I went to be tested for the the bio-markers for alz. It came back that i have Alz. My Mother had Alz. @ 54 years old ,lived to be 72. Im on aricept for at least 2mos. Im very interested in this study .My Neurologist wants me to wait until there are more information on this drug. My Insurance company hasn’t approved its use yet. Im open for any trial study.