Hours feel like days. Days feel like months. And months? Well they can feel like a decade. Particularly when you are awaiting the restart of a clinical trial of a drug that could mean a significant delay in developing Alzheimer’s.
That’s the situation I find myself in these days, waiting and waiting and waiting to be able to begin the redosing trial of Biogen’s experimental Alzheimer’s drug aducanumab, a controversial drug that has shown promise in treating the earliest stages of Alzheimer’s disease.
I truly believe that aducanumab was successful in slowing my symptoms, but the novel coronavirus is wreaking havoc on the development of the drug.
On Wednesday, Biogen said that its timeline for seeking FDA approval of aducanumab has been significantly delayed. The company initially estimated that it would file the necessary data in early 2020. In January, it told analysts that it would submit its application in a few weeks.
Of course, from a societal perspective, my waiting is small potatoes compared to those who have fought the disease or the family members of those who have passed away. And I’d never even think of comparing myself to the front-line heroes who are fighting the disease in our nation’s hospitals and nursing homes. Or the tens of millions of people who have lost their jobs. Even the people working at the local grocery stores, post offices and other essential businesses have it way worse.
All of that aside, I can’t help but feel sorry for myself, particularly as I lay awake at night riddled with anxiety about, well, everything: the pandemic, the economy and the disease slowly growing in my brain.
Yesterday, during a conference call to announce its quarterly earnings, Biogen said the application would not be submitted until the third quarter of this year. Third quarter seems pretty innocuous until you realize that is September … at the earliest.
During its earnings call, Biogen CEO Michel Vounatsos said that the company is “prioritizing quality of submission versus the timing.” And Al Sandrock, Biogen Research and Development Chief, assured investors that “nothing has come up in the data that changes our interpretation.”
Sandrock noted that Biogen itself had become ensnared in the coronavirus pandemic when a company meeting turned into one of the country’s first “super spreaders” of infection.
Quartz News reports that several financial analysts who previously expected the drug to be approved in 2020 changed their predictions to 2021 or 2022 after the earnings call.
Biogen, of course, is not the only company in the Alzheimer’s space experiencing disruptions. In an editorial published in the Journal of Prevention of Alzheimer’s Disease, two French scientists reported that the pandemic has severely impacted ongoing research. In March, the researchers said, that average weekly patient visits dropped to five from 52.
On a recent conference call with volunteer leaders, Maria Carrillo, chief scientist at the Alzheimer’s Association, noted that “research across the globe is on pause.”
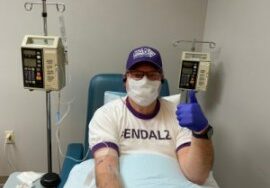
One lucky exception is Jeff Borghoff, a Being Patient advisor who in March became the first person in the world to begin the redosing study of aducanumab. Borghoff recently received his second dose of aducanumab (that’s him pictured above receiving his infusion in mask and gloves).
“I couldn’t see everyone’s smiling faces, although the positive demeanor and voices of the staff were shining through the obvious situation,” Borghoff wrote on his website. “There was still the hustle of the staff to care for a number of clients that were there for various protocols.”
So while I await getting started with the redosing trial, I’ll take solace that folks like Borghoff and the staff at Advanced Memory Research Institute of NJ, continue to persist in research that will ultimately help us find a treatment and cure for Alzheimer’s.
After all, heaven knows we’re all learning the value of patience. And while I wait, I’ll keep remembering the struggles and heroes of the coronavirus pandemic. Keeping them in my heart and trying to do my part to help those suffering, will, I’m certain, help the days go by more quickly.
Phil Gutis is a former New York Times reporter and current Being Patient senior reporter who was diagnosed with early onset Alzheimer’s. This article is part of the PHIL’S JOURNAL series, in which Phil chronicles his experience living with Alzheimer’s.