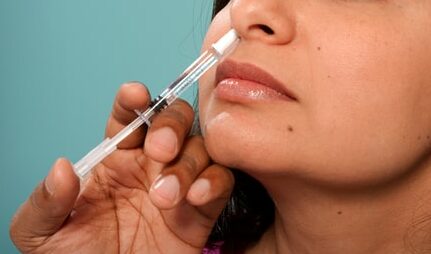
Human clinical trials kick off for Protollin, an experimental Alzheimer’s vaccine.
After two decades of research and development, Brigham and Women’s Hospital has initiated a Phase 1 trial of the Protollin Alzheimer’s nasal vaccine. The trial has recruited 16 people in the early stages of Alzheimer’s disease to participate in the trial. Researchers will follow them for six months to assess the candidate vaccine’s dosing and safety. Participants are between 60 and 85 years of age, with early symptomatic Alzheimer’s and beta-amyloid plaque buildup (one of Alzheimer’s key biomarkers) in their brains, according to PET scan brain imaging.
“What we’re doing, it’s never been done before,” Dr. Howard Weiner, co-director of the Ann Romney Center at Brigham and Women’s Hospital told WCVB news in Boston.
The team reportedly hopes to submit an application for approval from the Food and Drug Administration “in about five years.” Upon approval, the vaccine could move into manufacturing and then distribution to the general public. Currently, one in 10 people will develop Alzheimer’s by the age of 65. At age 85, chances leap to one in three.
Drugmakers I-Mab Biopharma and Jiangsu Nhwa Pharmaceutical are responsible for the development, manufacturing and commercialization of the new vaccine. Brigham and Women’s Hospital will administer this Phase 1 clinical trial to assess the safety and tolerability of the vaccine as well as its effect on white blood cells.
How does Protollin work?
Through the incredible power of our immune system, researchers are developing new drugs and treatments to tackle Alzheimer’s disease. Aduhelm, an anti-amyloid antibody-based drug was approved in June and other similar therapeutics, including gantenerumab, donanemab, and lecanemab, are currently in Phase 3 clinical trials, but these drugs require periodic intravenous infusions which can only be administered at certain medical facilities, creating serious barriers to access. Vaccines provide another immune-based strategy to potentially modify and prevent Alzheimer’s, and the hope is that they will be easier to distribute and more affordable to administer. There are about nine vaccines in various stages of clinical trials at this time.
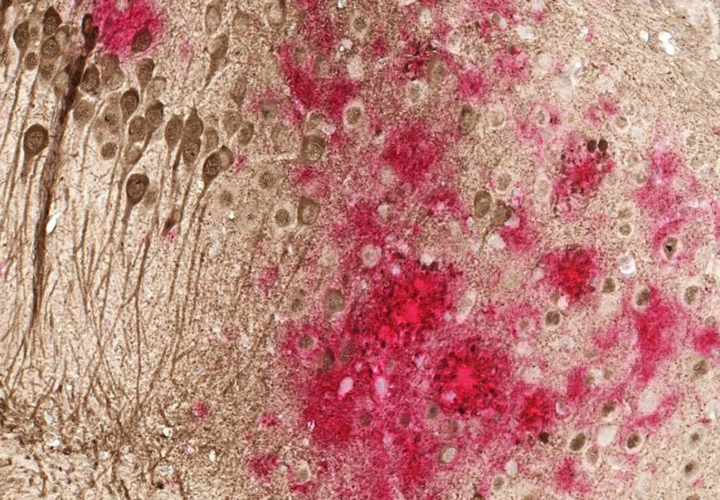
Some Alzheimer’s vaccines in the development pipeline target specific misfolded proteins that are known Alzheimer’s biomarkers, such as tau or beta-amyloid, like the aforementioned anti-amyloids do. Protollin, however, doesn’t target these Alzheimer’s proteins. Instead, it stimulates the innate immune system, which contains cells called macrophages and microglia that eat away debris.
For this reason, Dr. Tanuja Chitnis, physician, professor of neurology at the Brigham and Women’s Hospital, and principal investigator of the trial, told Being Patient that the approach could eventually prove useful for other diseases with misfolded proteins like Huntington’s or Parkinson’s disease.
“Only recently has the immune system been used to try to treat Alzheimer’s,” Chitnis said. “I think this is a brand new phase [for developing Alzheimer’s treatments], we saw this change occur in cancer treatments 20 years ago.”
“We think that in Alzheimer’s, these cells are not doing their job sufficiently,” she continued, noting that “protollin is made up of several proteins, one is an outer membrane protein from a bacteria called Neisseria.” This excites the innate immune system, she said, helping it to eat away amyloid plaques that have formed in the brain.
Will Protollin need boosters?
“In different mouse models, using the drug every few weeks can help to eliminate and reduce the beta-amyloid plaques,” Chitnis said. “It’s possible that this could be a drug that could be dosed once a month, or currently, we’re giving two doses over a two week period.” Vaccines and other drug treatments could prove synergistic, she added, proving more effective when combined: “There could be down the road room for two or more drugs to be used at the same time that are targeting different mechanisms in Alzheimer’s.”
What’s next for this Alzheimer’s nasal vaccine?
According to Chitnis, there aren’t currently any biomarkers consistently reliable, sensitive and specific enough to help diagnose Alzheimer’s before symptoms set in. But as scientists continue to search for better biomarkers in the blood, human eye and elsewhere, the researchers hope that in future trials, this vaccine and other candidates like it could be administered to people before they show any signs of Alzheimer’s disease, to help delay or prevent the disease altogether.