In the midst of a media frenzy around fraud allegations and a key Alzheimer’s drug target, reporter Phil Gutis reflects on his personal experience with the drug target in question — and takes off for AAIC.
Last week, with my anxiety levels already beginning to climb as I prepared to travel to the Alzheimer’s Association International Conference in San Diego, my inbox suddenly began to fill with messages about a great scientific fraud committed about 16 years ago by an Alzheimer’s researcher in Minnesota.
Thinking that the upcoming conference would be consumed with discussion of the alleged fraud, I immediately clicked over to the story in Science Magazine, scanned through looking for words that I could actually understand and ultimately came to the conclusion that it didn’t seem like there was much ‘there’ there.
I certainly did not feel like I found the fraud of the century. To simplify, it seemed that some researchers used Photoshop to muck with images that were published in a leading scientific journal back in 2006. Fraud, yes; bad, certainly; story of the century? Not from this layman’s perspective, limited as it may be.
In summary, the hype around the story was that because the researcher had used fraudulent images in a story about the role amyloid plays in the development of Alzheimer’s disease, the entire so-called amyloid hypothesis — perhaps the leading driver of research into the cause of Alzheimer’s for the past 20-plus years — could therefore be called into question. Turns out not so much.
As Edward Lee, a neuropathologist and associate director of the Penn Aging and Disease Resource Center, put it: “Just because one study may be problematic does not cause the entire field to collapse.” he said. “My read of the literature, with or without this one study, is that beta-amyloid remains one of several important factors in the pathogenesis of Alzheimer’s disease.”
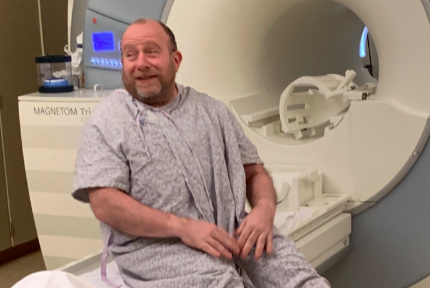
I’ve been engaged with the doctors at Penn ever since my diagnosis with early onset Alzheimer’s about six years ago. For a few years, I traveled to Penn from my suburban Philadelphia home once a month to participate in the Phase 3 trial for aducanumab (now known as Aduhelm), and I am lucky to consider the co-directors of Penn’s Memory Clinic my neurologists.
Aducanumab is one of those drugs that targets amyloid and, I’ve learned from the folks at Penn that after six years on the drug, I no longer have any amyloid in my brain. As readers may remember, I do believe that despite the controversy around Aduhelm, it has helped me a great deal.
(In February 2024, Biogen took Aduhelm off the market indefinitely.)
All this to say that, while I want to stay up to date on the latest science — and, as needed, latest scandal — in Alzheimer’s research, what the media is abuzz about doesn’t always align with my experience. This is why, whenever I can, I prefer to get my information directly from the experts.
Touching down at AAIC 2022
The upcoming Alzheimer’s Association International Conference in San Diego will mark the second scientific gathering I’ve covered as a reporter for Being Patient. My first was in 2019, when I traveled across the country to attend that year’s Clinical Trials in Alzheimer’s Disease conference – commonly known as CTAD – where drugmaker Biogen was unveiling some eagerly awaited trial data.
Writing about the experience for Being Patient, I acknowledged spending most of my time bewildered as the scientific crowds discussed concepts that I had no hope of following. So why was I there? In my journal, I noted that as I watched a Biogen Vice President spin out reams of data, I was more than a dispassionate observer. In fact, as a person living with an Alzheimer’s diagnosis, I was perhaps the most personally engaged attendee in the room.
Now, with my second reporting trip for Being Patient – and my first AAIC – just around the corner, I’m more than a bit anxious about trying to understand the science speak, but also eager to learn about the progress of thousands of scientists around the world who are trying to find a treatment or cure for this disease.
I’ll have a front-row seat as researchers present data next Tuesday on a grab bag of updates, deeper dives, preliminary and top-line results of various trials to find a treatment or cure for Alzheimer’s and other dementias.
The latest on Alzheimer’s drug trial findings
First up, researchers will take a deeper dive into a disappointing June 16 report on the Alzheimer’s Prevention Initiative Autosomal Dominant Alzheimer’s Disease Colombia Trial with crenezumab, a drug that was developed by Genentech and Roche. In that June report, the companies said that crenezumab ultimately failed to slow or prevent Alzheimer’s disease in a cohort of cognitively healthy people with early-onset Alzheimer’s genes. While the trial was unsuccessful, we’re supposedly going to learn what researchers drew from their years of work and how that knowledge may be applied to ongoing research.
Researchers will also provide early data on a new drug that seeks to improve dysfunctional glucose energy and lipid metabolism in Alzheimer’s disease. And conference-goers will get top-line results from the longest-ever Phase 3 study of the impact of exercise on cognition and brain function in older adults with mild cognitive impairment.
From COVID to CTE
Other topics being explored at the conference include new information on COVID and cognitive symptoms, particularly the impact of long COVID; racism, health disparities and their impact on cognition and dementia; and the latest in blood biomarkers and forthcoming blood-based Alzheimer’s diagnostics. We’ll also hear about preeclampsia and hypertension during pregnancy and the risk of developing vascular dementia. Another group of scientists will report on head injuries and chronic traumatic encephalopathy (CTE) and cognition.
Progress with Participant FIRST
And in a report that I’m particularly interested in, we’ll get the first set of 17 recommendations developed by a workgroup looking at how clinical trial sponsors, researchers and other staff can better communicate with study participants when clinical trials stop sooner than expected.
Currently, study participants and their families are generally the last to learn about the failure of clinical trials and they most typically receive the often-emotional information through the news or social media. To lessen the likelihood of market manipulation, publicly traded companies are required to release “market moving information,” such as the success or failure of a drug trial, to the public before telling insiders about the news. Thus, the news media and Wall Street traders typically get any significant news about drug trials before participants, family members or even the trial centers themselves.
On Tuesday, a workgroup cleverly titled Participant FIRST will take the first steps toward ending that imbalance as they outline what they’ve identified best practices for communicating with and supporting Alzheimer’s research volunteers and their study partners.
I’ve gotten an advance peek at their recommendations and I can say that, if adopted, they will represent progress. Here’s to hoping that isn’t the only time that AAIC attendees will hear of long-awaited progress.
Stay tuned here for updates.
Phil Gutis is a former New York Times reporter and current Being Patient contributor who was diagnosed with early onset Alzheimer’s. This article is part of his Phil’s Journal series, chronicling his experience living with Alzheimer’s and his participation in the aducanumab clinical trial.
UPDATE: 3 March 2024, 9:20 P.M. ET. In February 2024, Biogen took Aduhelm off the market, citing financial concerns. Although the drug did receive accelerated, conditional FDA approval for the treatment of early Alzheimer’s disease in 2021, it is no longer available to new patients. The company announced it would sunset trials in May 2024 and cease supplying the drug to current patients in November 2024.