New research takes one of the most widespread and fundamental theories about what causes Alzheimer's and turns it on its head: Now some scientists are asking, do we need to get rid of beta-amyloid? Or do we need to rebuild it?
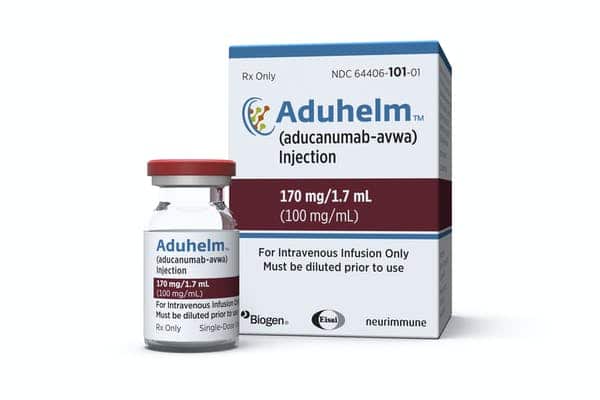
In the 1990s, the idea that clumps of beta-amyloid proteins are the main culprits of Alzheimer’s took off, and this line of thought — the amyloid hypothesis — has become the mainstay of drug development. Over roughly two decades, scientists have developed drugs that effectively clear the build-up of these proteins, known as amyloid plaques, but experts say these therapies have largely failed to benefit patients. In the case of Aduhelm (aducanumab), the efficacy of the FDA-approved plaque buster remains in question, placing the amyloid hypothesis front and center of debates about the agency’s Aduhelm approval decision.
There has never been a consensus on the amyloid hypothesis among the research community, and over the years, new findings have led to iterations of the idea. Now, a recent study has put into question the foundations of the theory: Rather than aiming to get rid of amyloid plaques, the researchers suggested that replenishing soluble fragments of beta-amyloid proteins (known as beta-amyloid peptides) may help slow neurodegeneration. These peptides help keep the brain healthy, and the scientists behind this new research said cognitive impairment may actually be due to the loss of beta-amyloid peptides, not the accumulation of amyloid plaques.
Dr. Alberto Espay, the study’s senior author and professor of neurology at the University of Cincinnati, sees amyloid plaques as the consequence of Alzheimer’s rather than the cause. These plaques are the tombstones of functioning beta-amyloid peptides, which are soluble in their normal state, according to the team. But guided by the amyloid hypothesis, Espay said researchers have pursued the idea that eliminating amyloid plaques can help patients, even though scientific evidence shows otherwise.
“This is the first time that the alternative to the prevailing hypothesis for Alzheimer’s is tested,” Espay told Being Patient of the recent study. “It had really never before been tested, largely because there has been an undying adherence to the amyloid hypothesis and honestly, a clear inability [on the part of] scientists to falsify the hypothesis, even in the context of consistently contrary evidence from many trials.” He added, “Any evidence to the contrary is discarded. Any indirect evidence that could favor it seems to be used to buttress it.”
Aduhelm’s Phase 3 clinical trials showed conflicting results, and while some researchers and patients say the drug may help with slowing decline, other experts criticized the FDA’s approval given the shoddy evidence of Aduhelm’s benefits. That said, the FDA’s accelerated approval of Aduhelm was based on the drug’s effect on clearing amyloid plaques, which the agency stated “is reasonably likely to predict a clinical benefit to patients.” By doing so, Espay said the agency has lowered the bar for other therapies in the pipeline, giving the impetus for companies with drugs that achieve what Aduhelm does — removing amyloid plaques — to seek an easier path of approval.
Following Aduhelm’s green light, two experimental anti-amyloid therapies, donanemab and lecanemab, earned the FDA’s breakthrough therapy status, a process that aims to accelerate the development and review of drugs for serious conditions. This shows that the field of Alzheimer’s will remain heavily invested into anti-amyloid approaches in the near future, Espay explained, crowding out resources from other strategies to develop Alzheimer’s treatments.
In the study published in EClinicalMedicine, Espay and colleagues probed the link between the loss of soluble beta-amyloid peptides (Aβ42), amyloid plaques and cognition. The team analyzed data of 598 people enrolled in the Alzheimer’s Disease Neuroimaging Initiative study, all of whom had elevated levels of amyloid plaques in their brain.
Regardless of the amount of amyloid plaques, the team found that people with normal cognition were more likely to have higher levels of soluble beta-amyloid peptides, compared to those with mild cognitive impairment or dementia.
Higher levels of these soluble peptides were also associated with better cognitive performance and greater volume of the hippocampus, the brain’s memory center.
According to Espay, soluble beta-amyloid peptides play a host of important roles, including to help keep neurons healthy and to facilitate their communication. They have also been shown to be antimicrobial peptides, helping fend off invading microorganisms such as bacteria in the brain.
In Alzheimer’s, however, these peptides begins accumulating between neurons and becomes insoluble. The peptide’s structure falters as it misfolds, unable to carry out its tasks. With increasing aggregation, fewer functional peptides are available, eventually leading to symptoms of Alzheimer’s.
Espay and team are now testing therapies to boost soluble beta-amyloid peptides and other proteins in animal models, examining the drugs’ safety and effects at the cellular and behavioral levels. w
He noted that scientists have billed proteins associated with degenerative brain diseases like Alzheimer’s, or for that matter, Parkinson’s, as the “the central villains in the drama of neurodegeneration,” when in fact, the loss of these normally functioning proteins may actually be the problem.
“We have overlooked what is certain: We know for certain that proteins can only function when they’re normal … But we’ve embraced the uncertainty that once they clump, somehow they turn to toxins. That really is just our attempt to [create] a narrative that seems logical, compelling, fascinating, but ultimately, biologically completely fictional,” he said.
“Because it is much more compelling of a narrative to have a central villain, it’s kind of hard to remove from it, even though the data doesn’t support it,” he added. “If anything, the data has actually suggested the opposite.”
Ultimately, Espay said factors involved in the misfolding of proteins likely differ from one person to another. Just like degenerative brain diseases such as Parkinson’s, Espay believes that Alzheimer’s isn’t just one disease: It likely consists of different subtypes depending on a person’s specific biological abnormalities.
In search for effective treatments, Espay and colleagues are carrying out research to identify and target the unique biology of individuals with neurodegenerative diseases through precision medicine.


Que interesante gracias doctor Alberto Spay, que siga progresando la investigacion y aparezca la cura para esta terrible enfermedad