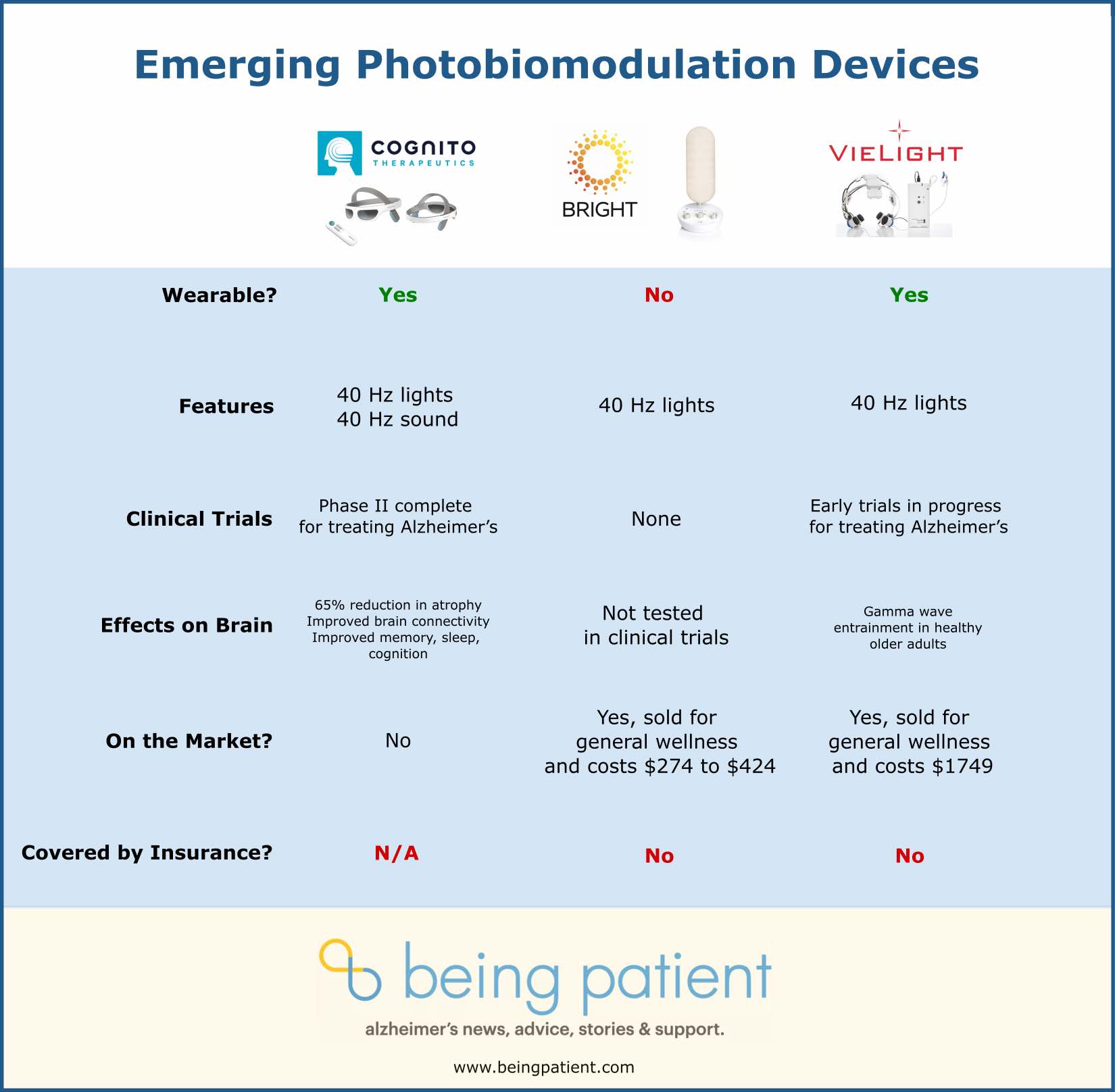
IMAGE: Photobiomodulation tabletop device Beacon40 by neurotechnology company Bright
Could flickering lights have brain health benefits? Research around experimental therapies involving external stimuli — like light and sound — is growing. And some devices are already hitting the market, while others are entering clinical trials to prove their efficacy. Being Patient reporter Simon Spichak looks at what we know about each device and the science at large.
One of the challenges in Alzheimer’s disease (AD) drug development is finding treatments that are actually capable of reaching the brain. The blood-brain barrier, a border between the brain and the rest of the body, is impenetrable to many contaminants or intruders, making it difficult to design a therapeutic to sneak across. But what if a treatment could be delivered directly to the brain?
Innovations in neuroscience and neurotechnology are creating new avenues for non-invasive therapies for Alzheimer’s with this challenge in mind. And increasingly, scientists and clinicians are exploring new technologies that skirt the blood-brain barrier, leveraging light and sound stimulation to access the brain directly.
From this pursuit has emerged a new niche of the neurotechnology industry, with a focus on devices that emit flashing light designed to help boost brain health or even, possibly, treat neurodegeneration. Some have earned FDA breakthrough status while others are available on the market now.
How Does Flickering Light Therapy Work?
Scientists have been studying the health benefits of different types of light stimulation for decades; there are two general approaches — neuromodulation through visible wavelengths of light that activate the optic nerve and photobiomodulation where near-infrared wavelengths of light penetrate into cells, thereby exerting beneficial effects. The latter, photobiomodulation, utilizes non-ionizing forms of light sources including lasers, LEDs, and broadband light to treat things like pain relief and inflammation.
Gamma brain waves are produced by your brain’s firing neurons. The more intensely you are focused or the more engaged you are in solving a problem, the more gamma wave activity is happening inside your head. These waves are not only a sign of healthy neurons, they are also good for the brain.
In mice, visible light neuromodulation stimulates the brain’s innate immune cells and cleanup crew, the microglia, which help clean up beta-amyloid plaques — a hallmark of Alzheimer’s disease. In humans, as we discuss below, wearable devices that deliver 40 Hz visible light and aural stimulation may slow the progression of Alzheimer’s disease and improve symptoms.
Similarly, a photobiomodulation approach currently entering clinical trials may entrain gamma waves in the brain of older adults through intranasal and transcranial photobiomodulation, at 40 Hz. If there is a lack of gamma wave activity in Alzheimer’s and cognitive impairment, perhaps restoring this activity could treat some of the symptoms.
Neuromodulation delivered directly to the eyes via goggles and photobiomodulation, both transcranial and intranasal, are being explored as safe, minimally invasive ways to boost brain health or even, possibly, treat neurodegeneration.
Many of these claims are yet untested in human trial, and the sheer premise of treating Alzheimer’s or cognitive impairment with flickering lights and rhythmic tones draws a lot of skepticism from neurological researchers. Yet, preclinical data from studies in mice and cells has encouraged their makers to push further, with some devices made available before clinical testing and trials.
While some devices, like the wearable Cognito Tx and Vielight Neuro RX Gamma, designed for Alzheimer’s patients, would require a prescription if they make it through trials to market, others like the Vielight Neuro Gamma and the tabletop device Bright Beacon40 are newly available on the market for anyone to purchase and use for general cognitive health.
Being Patient set out to examine three companies’ emerging consumer technologies, looking at how they work, and the evidence behind their efficacy. These devices are still in the early stages, and their benefits have not yet been shown in Phase 3 clinical trials.
Cognito Therapeutics
What it’s for: Designed to treat Alzheimer’s
Current availability: In clinical trials, received FDA breakthrough status in 2021
The Cognito Tx is a wearable device — like a pair of glasses — that delivers both light and sound to the brain. The lenses on the glasses are surrounded by small lights emitting gamma frequency stimulation. When the light hits the optic nerve, it is converted to electrical signals in the brain’s visual cortex. The built-in hardware adjusts the intensity, frequency, and duration of the signal in response to any movement. This ensures that gamma frequency-encoding light is continually delivered to the periphery.
In a recent Phase 2 double-blind clinical control trial of mild-to-moderate Alzheimer’s, their device reduced the amount of brain volume lost over the course of six months. In an interview with Being Patient, Brent Vaughan, CEO at Cognito Therapeutics explained the treatment regimen. “In our clinical studies, we’ve had the patients using it one hour a day, at home on a daily basis.”
Cognito Therapeutics is backed by both preclinical and clinical data, showing its effectiveness. It is the only one of these devices that has conducted a Phase 2 trial — which found that their device reduced the loss of brain volume by 65 percent while improving memory, sleep, and cognition in people with Alzheimer’s.
“When we turn on the gamma frequency light stimulation, we start seeing gamma frequency entrainment across the brain. We’ve shown that that leads to microglia activation and increased synaptic density and connections,” Vaughan told Being Patient. “We had over 90 percent compliance of people actually using it as instructed. At the end of six months, we gave everyone a chance to continue using the device or stop the study. And almost 85 percent of the people elected to continue using it.”
The flickering lights and tones activated the brain’s microglia, allowing them to clear beta-amyloid and prevent cell death while maintaining connections between individual brain cells. The company, which received an FDA Breakthrough Device designation in January 2021 will soon start Phase 3 clinical trials.
Bright Beacon40
What it’s for: Designed to improve cognition, sleep and memory.
Cost and availability: The Beacon40 is available for purchase through Bright’s website with a price range from $274 for a single light and $434 for a pair. It is not covered by insurance.
Bright’s device emits a flashing light, at gamma frequencies, from a small lamp. Unlike the wearable Cognito Therapeutics and Vielight devices, the Beacon40 is a stand-alone product, like a lamp. It also does not use sound. The device allows a user to set the color, tone, and brightness of the light, as well as its duration.
The Beacon40 is not considered a medical device. It hasn’t been tested to determine whether a lamp is just as effective as a headset for gamma frequency delivery. But, according to the product’s website, it is designed to improve memory, sleep, cognition, and more. There are no studies to indicate over what timeframe those changes might take place.
Bright provided us with a sample of the product to test it out ourselves, which we returned afterward. You can view our demonstration below. I used the device a few times for an hour per session — too short to notice whether it made me feel any different.
Conducting market research, their team found that potential users with early-stage dementia or at risk for Alzheimer’s preferred a lamp to a headset. While lamps haven’t been investigated in humans through clinical trials, the lamp was used successfully in a mouse model of Alzheimer’s, activating microglia and leading to a reduction in beta-amyloid plaques.
But while the Beacon40 provides a sleek design, the company has not conducted any trials to assess whether it increases gamma frequency oscillations in the brain. Wendy Bronfin, chief product and marketing officer, explained why their company, Bright, built the device after reading about preclinical research. “For people like us with family members who are battling Alzheimer’s disease, we’re running a race against time. We don’t have the luxury to wait for FDA approval of either a medication or a photobiotic biomodulation technology. You’ve got to act now.”
Bronfin told Being Patient that several users reported a positive impact from using the light; however, they are not planning on conducting clinical trials. Ultimately, there is no vetted scientific research supporting the efficacy of this particular device.
Vielight Neuro Gamma
What it’s for: Designed to increase gamma waves in the brain, which are associated with cognition.
Cost and availability: The Vielight Neuro Gamma is available for $1,749 through Vielight’s website. It is not covered by insurance.
Vielight produces wearable headsets that provide near-infrared light stimulation, using a photobiomodulation approach. The device has LED diodes that clip into the nose, as well as in the ears. None of the light is directed to the eyes, making the light delivery less distracting than devices that may direct the light through the eyes. Wearing this device for twenty minutes a day may help improve general brain health and cognition. The Neuro Gamma is currently recognized as a low-risk general wellness device by the FDA but is not tested for any specific health claims.
Lew Lim, founder and CEO of Vielightm explained in a recent video interview with Being Patient, “If we want to make a claim for Alzheimer’s disease, we have to produce the evidence by going through the regulatory process.”
In order to comply with current policy, the Neuro RX Gamma is being tested in clinical trials, and cannot be sold without regulatory clearance.
Watch the full LiveTalk here.
Vielight Neuro RX Gamma
What it’s for: Designed to treat Alzheimer’s
Cost and availability: Neuro RX Gamma, is not currently available. It is in clinical trials with FDA breakthrough status
Vielight’s Neuro RX Gamma is currently being tested in an interventional clinical trial for Alzheimer’s to assess whether the device improves the cognitive and physical symptoms of Alzheimer’s. Scientists have previously tested this device in healthy older adults, finding that it is able to increase gamma frequency brain waves.
Francisco Gonzalez-Lima, a professor of anatomy at the University of Texas at Austin medical school, emphasized the need for more evidence that these devices work. He hasn’t seen compelling evidence to suggest that 40 Hz photobiomodulation has any different or better effects, he said. “From a theoretical standpoint, 40 Hz photobiomodulation would not induce different effects than continuous wave, because of the scattering of light that happens when light goes through the head, making pulsing and continuous wave light similarly spread in the head,” he told Being Patient.
Both Gonzalez-Lima and another expert, Michael Hamblin, a professor in the Laser Research Center and faculty of Health Sciences at the University of Johannesburg, discussed the relevance of a device’s wattage output in how effective its photobiomodulation effort may be in improving cognitive health.
When it comes to the goal of using photobiomodulation to treat Alzheimers, Gonzalez-Lima added that implying these devices can treat Alzheimer’s now may inspire “false hope” due to the lack of existing, established research: “There are no published, randomized, controlled human studies of photobiomodulation for effective cognitive augmentation or neuroprotection in Alzheimer’s disease,” he said. “There are only case reports, anecdotes, and overstated claims, without randomized controlled human studies of AD.”
In Lim’s talk, he mentioned that preliminary evidence showed improvements in blood flow to the brain as well as brain fog. However, he emphasized the need for more research in this space, especially across different severities of Alzheimer’s disease.
“Our clinical trial involves 218 subjects with Alzheimer’s disease, not just mild but moderate to severe,” Lim said. “Caregivers want innovators to take the plunge, and test in severe cases.”
CORRECTION SEPT. 7, 2021: A previous version of this article applied the term photobiomodulation incorrectly. This article has been updated to distinguish between photobiomodulation — using certain types of light to trigger changes in structures within cells — and neuromodulation — using sensory stimuli including light to activate nerve cells.


If light therapy worked, it would be fabulous!! We need to find a cure for this hideous disease…
This is great news “light therapy “ for Alzheimer’s. What’s the hold up?
Are there not enough people to do a study? I’m 59 and a recent PET scan showed amyloid plaque on my brain. I’ve been having memory issues for a couple years and it’s getting worse.
I’m in a study but I don’t know if I will get the placebo or the real trial medication. I’m scared and sad.
Hi Cynthia! One of the companies we reported on called Cognito Therapeutics is currently finishing up a Phase 2 trial, testing a head set that emits a specific wavelength of light and sound to help treat Alzheimer’s. However, it takes a long time to go through these clinical trials, and it may take several more years to get more data and research in humans before the company can apply for approval from the FDA. Sending you hope and strength as you navigate this.